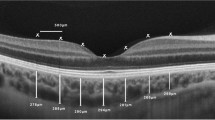
Abstract
Purpose
Retinal and choroidal microvascular changes can be related to renal impairment in hypertension and chronic kidney disease (CKD). The study examines the association between retinochoroidal parameters and renal impairment in hypertensive, non-diabetic patients.
Methods
This is a cross-sectional study on Caucasian patients with systemic arterial hypertension with different levels of renal function. All subjects were studied by blood chemistry, urine examination, microalbuminuria and blood pressure. Complete eye examination was completed with swept source optical coherence tomography (SS-OCT) and optical coherence tomography angiography (OCTA) scans of macular region. Patients were divided in groups: LowGFR and HighGFR and CKD− and CKD+, according to the value of glomerular filtrate (GFR) and albuminuria. LowGFR and CKD+ groups included patients with clinical kidney impairment.
Results
One hundred and twenty eyes of 120 hypertensive patients were evaluated. The mean retinal thickness was thinner in CKD+ versus CKD− group (p < 0.009). LowGFR and CKD+ groups showed thinner choroidal values than HighGFR (p < 0.02) and CKD− (p < 0.001) groups. OCTA showed lower density in LowGFR than in HighGFR group (p < 0.001) and in CKD+ versus CKD− group (p < 0.001). Albuminuria was inversely related to choroidal and retinal thickness measures (p < 0.001) and to the indices of superficial parafoveal (p < 0.05) and foveal (p < 0.05) vascular densities.
Conclusions
CKD is associated with retinal thinning, eGFR and decreasing renal function with progressive reduction of choroidal and retinal vascular density. SS-OCT and OCTA documented close association between CKD and reduction of both choroidal thickness and paracentral retinal vascular density in hypertensive patients.





Similar content being viewed by others
References
Jorgensen T, Capewell S, Prescott E et al (2013) Population-level changes to promote cardiovascular health. Eur J Prev Cardiol 20(3):409–421
Mendis S, Davis S, Norrving B (2015) Organizational update: the world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke 46(5):e121–e122
Lewington S, Clarke R, Qizilbash N, Prospective Studies Collaboration et al (2002) Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360(9349):1903–1913
Mulè G, Castiglia A, Cusumano C et al (2017) Subclinical kidney damage in hypertensive patients: a renal window opened on the cardiovascular system. Focus on microalbuminuria. Adv Exp Med Biol 956:279–306
Wong CW, Wong TY, Cheng CY et al (2014) Kidney and eye diseases: common risk factors, etiological mechanisms, and pathways. Kidney Int 85(6):1290–1302
Wilkinson-Berka JL, Agrotis A et al (2012) The retinal renin-angiotensin system: roles of angiotensin II and aldosterone. Peptides 36:142–150
Benigni A, Cassis P, Remuzzi G (2010) Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med 2:247–257
Wong TY, Coresh J, Klein R et al (2004) Retinal microvascular abnormalities and renal dysfunction: the atherosclerosis risk in communities study. J Am Soc Nephrol 15(9):2469–2476
Grunwald JE, Alexander J, Maguire M, CRIC Study Group et al (2010) Prevalence of ocular fundus pathology in patients with chronic kidney disease. Clin J Am Soc Nephrol 5(5):867–873
Ferrara D, Waheed NK, Duker JS (2016) Investigating the choriocapillaris and choroidal vasculature with new optical coherence tomography technologies. Prog Retin Eye Res 52:130–155
ESH/ESC task Force for the Management of Arterial Hypertension (2013) 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC task force for the management of arterial hypertension. J Hypertens 31(10):1925–1938
Downie LE, Hodgson LA, Dsylva C et al (2013) Hypertensive retinopathy: comparing the Keith-Wagener-Barker to a simplified classification. J Hypertens 31(5):960–965
Balmforth C, van Bragt JJ, Ruijs T et al (2016) Chorioretinal thinning in chronic kidney disease links to inflammation and endothelial dysfunction. JCI Insight 8(1):20 e89173
Mulè G, Vadalà M, Geraci G, Cottone S (2018) Retinal vascular imaging in cardiovascular medicine: new tools for an old examination. Atherosclerosis. 268:188–190. https://doi.org/10.1016/j.atherosclerosis.2017.11.001
Nickla DL, Wallman J (2010) The multifunctional choroid. Prog Retin Eye Res 29:144–168
Linsenmeier RA, Padnick-Silver L (2000) Metabolic dependence of photoreceptors on the choroid in the normal and detached retina. Invest Ophthalmol Vis Sci 41:3117–3123
Spraul CW, Lang GE, Lang GK et al (2002) Morphometric changes of the choriocapillaris and the choroidal vasculature in eyes with advanced glaucomatous changes. Vis Res 42:923–932
Brown JS, Flitcroft DI, Ying G et al (2009) In vivo human choroidal thickness measurements: evidence for diurnal fluctuations. Invest Ophthalmol Vis Sci 50:5–12
Gao SS, Jia Y, Zhang M et al (2016) Optical coherence tomography angiography. Invest Ophthalmol Vis Sci 57(9):OCT27–OCT36
Cerasola G, Cottone S, Mule G (2010) The progressive pathway of microalbuminuria: from early marker of renal damage to strong cardiovascular risk predictor. J Hypertens 28(12):2357–2369
Chen YH, Chen HS, Tarng DC (2012) More impact of microalbuminuria on retinopathy than moderately reduced GFR among type 2 diabetic patients. Diabetes Care 35(4):803–808
Schlaich MP, Socratous F, Hennebry S et al (2009) Sympathetic activation in chronic renal failure. J Am Soc Nephrol 20(5):933–939
Wallman J, Wildsoet C, Xu A et al (1995) Moving the retina: choroidal modulation of refractive state. Vis Res 35(1):37–50
Copete S, Flores-Moreno I, Montero JA et al (2014) Direct comparison of spectral-domain and swept-source OCT in the measurement of choroidal thickness in normal eyes. Br J Ophthalmol 98(3):334–338
Bahrami B, Ewe SYP, Hong T et al (2017) Influence of retinal pathology on the reliability of macular thickness measurement: a comparison between optical coherence tomography devices. Ophthalmic Surg Lasers Imaging Retina 48(4):319–325
Ulaş F, Doğan Ü, Keleş A et al (2013) Evaluation of choroidal and retinal thickness measurements using optical coherence tomography in non-diabetic haemodialysis patients. Int Ophthalmol 33(5):533–539
Jung JW, Chin HS, Lee DH et al (2014) Changes in subfoveal choroidal thickness and choroidal extravascular density by spectral domain optical coherence tomography after haemodialysis: a pilot study. Br J Ophthalmol 98(2):207–212
Ruiz-Medrano J, Flores-Moreno I, Peña-García P et al (2014) Macular choroidal thickness profile in a healthy population measured by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci 55(6):3532–3542
Masis M, Hernandez E, Wu L (2011) Choroidal thickness in patients with systemic hypertension. ARVO Meeting Abstract Invest Ophthalmol Vis Sci (52):5296
Akay F, Gundogan FC, Yolcu U et al (2016) Choroidal thickness in systemic arterial hypertension. Eur J Ophthalmol 26(2):152–157
Liao MT, Sung CC, Hung KC et al (2012) Insulin resistance in patients with chronic kidney disease. J Biomed Biotechnol 2012:691369
Chen J, Muntner P, Hamm LL et al (2003) Insulin resistance and risk of chronic kidney disease in nondiabetic US adults. J Am Soc Nephrol 14:469–477
Acknowledgements
Thanks are due to Sergio Milletarì, University of Palermo, for the clinical and technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No author has a proprietary interest in this study.
Maria Vadalà is consultant for Allergan plc Italia, Bayer SpA, Novartis Co.
Massimo Castellucci, Giulia Guarrasi, Micol Terrasi, Tiziana La Blasca and Giuseppe Mulè have no financial disclosures.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vadalà, M., Castellucci, M., Guarrasi, G. et al. Retinal and choroidal vasculature changes associated with chronic kidney disease. Graefes Arch Clin Exp Ophthalmol 257, 1687–1698 (2019). https://doi.org/10.1007/s00417-019-04358-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04358-3