Abstract
Background
The application of three-dimensional (3D) visualization techniques to evaluate the earliest visible onset of abnormal retinal vascular development in preterm infants with retinopathy of prematurity (ROP), using bedside non-contact optical coherence tomography (OCT) imaging to characterize morphology and sequential structural changes of abnormal extraretinal neovascularization.
Methods
Thirty-one preterm infants undergoing routine ROP screening with written informed consent for research imaging were enrolled in this prospective observational study. We imaged the macula and temporal periphery of preterm infants using a handheld OCT system (Envisu 2300 or handheld swept-source research system). The scans obtained were segmented and, using enhanced ray casting, were converted to 3D volumes to which color filter was applied.
Results
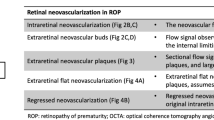
Using colorized 3D visualization, we defined extraretinal neovascular structures as buds, bridging networks, and placoid lesions. We could longitudinally follow progression and regression of extraretinal neovascularization in stage 3 ROP after treatment in one infant over 12 weeks and document the appearance of early buds, and formation of florid neovascularization. From stages 2 to 3 ROP, we observed progression from sessile buds to a complex plaque that corresponded to stage 3 ROP on clinical examination. We demonstrated regression of neovascular complexes to small pre-retinal tufts after treatment with anti-VEGF.
Conclusions
The extension of OCT processing to include surface flattening and colorization that further improved structural analysis rendered better understanding of extraretinal tissue. Our ability to image similar areas in the same infant over multiple visits enabled us to study the evolution of these structural components and follow pathological vascular events longitudinally in development and regression after treatment. These methods can be applied to further study which are likely contribute to our understanding of the pathophysiology of neovascularization in ROP.






Similar content being viewed by others
References
Gilbert C, Foster A (2001) Childhood blindness in the context of VISION 2020--the right to sight. Bull World Health Organ 79:227–232
Hartnett ME (2010) The effects of oxygen stresses on the development of features of severe retinopathy of prematurity: knowledge from the 50/10 OIR model. Doc Ophthalmol 120:25–39. https://doi.org/10.1007/s10633-009-9181-x
Mann IC (1928) The develpoment of the human eye. Cambridge University Press, London
Hartnett ME (2015) Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology 122:200–210. https://doi.org/10.1016/j.ophtha.2014.07.050
Hartnett ME (2017) Advances in understanding and management of retinopathy of prematurity. Surv Ophthalmol 62:257–276. https://doi.org/10.1016/j.survophthal.2016.12.004
Lutty GA, McLeod DS, Bhutto I, Wiegand SJ (2011) Effect of VEGF trap on normal retinal vascular development and oxygen-induced retinopathy in the dog. Invest Ophthalmol Vis Sci 52:4039–4047. https://doi.org/10.1167/iovs.10-6798
Lutty GA, Chan-Ling T, Phelps DL, Adamis AP, Berns KI, Chan CK, Cole CH, D'Amore PA, Das A, Deng WT, Dobson V, Flynn JT, Friedlander M, Fulton A, Good WV, Grant MB, Hansen R, Hauswirth WW, Hardy RJ, Hinton DR, Hughes S, McLeod DS, Palmer EA, Patz A, Penn JS, Raisler BJ, Repka MX, Saint-Geniez M, Shaw LC, Shima DT, Smith BT, Smith LE, Tahija SG, Tasman W, Trese MT (2006) Proceedings of the third international symposium on retinopathy of prematurity: an update on ROP from the lab to the nursery (November 2003, Anaheim, California). Mol Vis 12:532–580
Foos RY (1987) Retinopathy of prematurity. Pathologic correlation of clinical stages. Retina 7:260–276
Foos RY, Kopelow SM (1973) Development of retinal vasculature in paranatal infants. Surv Ophthalmol 18:117–127
Ashton N, Cook C (1954) Direct observation of the effect of oxygen on developing vessels: preliminary report. Br J Ophthalmol 38:433–440
Ashton N (1954) Pathological basis of retrolental fibroplasia. Br J Ophthalmol 38:385–396
Schmidt-Erfurth U, Leitgeb RA, Michels S, Povazay B, Sacu S, Hermann B, Ahlers C, Sattmann H, Scholda C, Fercher AF, Drexler W (2005) Three-dimensional ultrahigh-resolution optical coherence tomography of macular diseases. Invest Ophthalmol Vis Sci 46:3393–3402. https://doi.org/10.1167/iovs.05-0370
Maldonado RS, Izatt JA, Sarin N, Wallace DK, Freedman S, Cotten CM, Toth CA (2010) Optimizing hand-held spectral domain optical coherence tomography imaging for neonates, infants, and children. Invest Ophthalmol Vis Sci 51:2678–2685. https://doi.org/10.1167/iovs.09-4403
Maldonado RS, Yuan E, Tran-Viet D, Rothman AL, Tong AY, Wallace DK, Freedman SF, Toth CA (2014) Three-dimensional assessment of vascular and perivascular characteristics in subjects with retinopathy of prematurity. Ophthalmology 121:1289–1296. https://doi.org/10.1016/j.ophtha.2013.12.004
Chavala SH, Farsiu S, Maldonado R, Wallace DK, Freedman SF, Toth CA (2009) Insights into advanced retinopathy of prematurity using handheld spectral domain optical coherence tomography imaging. Ophthalmology 116:2448–2456. https://doi.org/10.1016/j.ophtha.2009.06.003
Viehland C, Keller B, Carrasco-Zevallos OM, Nankivil D, Shen L, Mangalesh S, Viet d T, Kuo AN, Toth CA, Izatt JA (2016) Enhanced volumetric visualization for real time 4D intraoperative ophthalmic swept-source OCT. Biomed Opt Express 7:1815–1829. https://doi.org/10.1364/boe.7.001815
Bleicher ID, Jackson-Atogi M, Viehland C, Gabr H, Izatt JA, Toth CA (2018) Depth-Based, Motion-Stabilized Colorization of Microscope-Integrated Optical Coherence Tomography Volumes for Microscope-Independent Microsurgery. Translational Vision Science and Technology In Press
Hughes S, Yang H, Chan-Ling T (2000) Vascularization of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci 41:1217–1228
Smith LE, Hard AL, Hellström A (2013) The biology of retinopathy of prematurity: How Knowledge of Pathogenesis Guides Treatment. Clin Perinatol 40:201–214. https://doi.org/10.1016/j.clp.2013.02.002
Hartnett ME, Penn JS (2012) Mechanisms and management of retinopathy of prematurity. N Engl J Med 367:2515–2526. https://doi.org/10.1056/NEJMra1208129
Lepore D, Quinn GE, Molle F, Baldascino A, Orazi L, Sammartino M, Purcaro V, Giannantonio C, Papacci P, Romagnoli C (2014) Intravitreal bevacizumab versus laser treatment in type 1 retinopathy of prematurity: report on fluorescein angiographic findings. Ophthalmology 121:2212–2219. https://doi.org/10.1016/j.ophtha.2014.05.015
Purcaro V, Baldascino A, Papacci P, Giannantonio C, Molisso A, Molle F, Lepore D, Romagnoli C (2012) Fluorescein angiography and retinal vascular development in premature infants. J Matern Fetal Neonatal Med 25(Suppl 3):53–56. https://doi.org/10.3109/14767058.2012.712313
Garoon I, Epstein G, Segall M, Rabb MF, LaFranco F, Quirk TC 3rd (1980) Vascular tufts in retrolental fibroplasia. Ophthalmology 87:1128–1132
Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161:1163–1177. https://doi.org/10.1083/jcb.200302047
Kushner BJ, Essner D, Cohen IJ, Flynn JT (1977) Retrolental Fibroplasia. II Pathologic correlation. Arch Ophthalmol 95:29–38
Wallace DK, Kylstra JA, Greenman DB, Freedman SF (1998) Significance of isolated neovascular tufts ("popcorn") in retinopathy of prematurity. J Aapos 2:52–56
Chen X, Mangalesh S, Dandridge A, Tran-Viet D, Wallace DK, Freedman SF, Toth CA (2018) Spectral-domain OCT findings of retinal vascular-avascular junction in infants with retinopathy of prematurity. Ophthalmology Retina. https://doi.org/10.1016/j.oret.2018.02.001
Lepore D, Molle F, Pagliara MM, Baldascino A, Angora C, Sammartino M, Quinn GE (2011) Atlas of fluorescein angiographic findings in eyes undergoing laser for retinopathy of prematurity. Ophthalmology 118:168–175. https://doi.org/10.1016/j.ophtha.2010.04.021
Cunningham S, Fleck BW, Elton RA, McIntosh N (1995) Transcutaneous oxygen levels in retinopathy of prematurity. Lancet 346:1464–1465
Penn JS, Tolman BL, Lowery LA, Koutz CA (1992) Oxygen-induced retinopathy in the rat: hemorrhages and dysplasias may lead to retinal detachment. Curr Eye Res 11:939–953
Simmons AB, Bretz CA, Wang H, Kunz E, Hajj K, Kennedy C, Yang Z, Suwanmanee T, Kafri T, Hartnett ME (2018) Gene therapy knockdown of VEGFR2 in retinal endothelial cells to treat retinopathy. Angiogenesis. https://doi.org/10.1007/s10456-018-9618-5
Becker S, Wang H, Simmons AB, Suwanmanee T, Stoddard GJ, Kafri T, Hartnett ME (2018) Targeted knockdown of overexpressed VEGFA or VEGF164 in Muller cells maintains retinal function by triggering different signaling mechanisms. Sci Rep 8:2003. https://doi.org/10.1038/s41598-018-20278-4
McCloskey M, Wang H, Jiang Y, Smith GW, Strange J, Hartnett ME (2013) Anti-VEGF antibody leads to later atypical intravitreous neovascularization and activation of angiogenic pathways in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci 54:2020–2026. https://doi.org/10.1167/iovs.13-11625
Wang H, Yang Z, Jiang Y, Flannery J, Hammond S, Kafri T, Vemuri SK, Jones B, Hartnett ME (2014) Quantitative analyses of retinal vascular area and density after different methods to reduce VEGF in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci 55:737–744. https://doi.org/10.1167/iovs.13-13429
Acknowledgements
The authors would like to thank Du Tran-Viet and Alexandria Dandridge (Duke University Eye Center), for data acquisition, Dr. Neeru Sarin, MBBS (Duke University Eye Center), and Dr. C Michael Cotten (Department of Neonatology, Duke University Medical Center) for recruiting infants for the study. We also thank Colin A. Bretz, PhD (Moran Eye Center, Utah) for preparing, staining, and imaging the retinal flat-mount of the rat OIR model.
Funding
Bayer global ophthalmology awards program (GOAP) fellowship project award, K23EY028227 and RPB career development award (XC); The Hartwell Foundation (CAT); The Andrew Family Charitable Foundation (CAT); Grants P30 EY001583, RO1 EY025009 and R01 EY015130 and R01 EY017011 from the National Eye Institute (NEI); Rockefeller Writing Residency (CAT); and an Unrestricted Grant from Research to Prevent Blindness, Inc., New York, NY, to the Department of Ophthalmology & Visual Sciences, University of Utah. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NEI, or NIH. The sponsors or funding organizations had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was conducted under two study protocols and was approved by the Duke University Medical Center institutional review board and adhered to the tenets of the Declaration of Helsinki.
Conflict of interest
Dr. Toth receives royalties through her university from Alcon. Dr. Izatt has a patent and receives royalties from Leica Microsystems. No other authors have financial disclosures. No authors have a proprietary interest in the current study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants (parent/guardian) included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2666 kb)
Rights and permissions
About this article
Cite this article
Mangalesh, S., Bleicher, I.D., Chen, X. et al. Three-dimensional pattern of extraretinal neovascular development in retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 257, 677–688 (2019). https://doi.org/10.1007/s00417-019-04274-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04274-6