Abstract
Purpose
To evaluate the effectiveness of an ab interno subconjunctival gelatin implant as primary surgical intervention in reducing intraocular pressure (IOP) and IOP-lowering medication count in medically uncontrolled moderate primary open-angle glaucoma (POAG).
Methods
In this prospective, non-randomized, open-label, multicenter, 2-year study, eyes with medicated baseline IOP 18–33 mmHg on 1–4 topical medications were implanted with (phaco + implant) or without (implant alone) phacoemulsification. Changes in mean IOP and medication count at months 12 (primary outcomes) and 24, clinical success rate (eyes [%] achieving ≥ 20% IOP reduction from baseline on the same or fewer medications without glaucoma-related secondary surgical intervention), intraoperative complications, and postoperative adverse events were assessed.
Results
The modified intent-to-treat population included 202 eyes (of 218 implanted). Changes (standard deviation) in mean IOP and medication count from baseline were − 6.5 (5.3) mmHg and − 1.7 (1.3) at month 12 and − 6.2 (4.9) mmHg and − 1.5 (1.4) at month 24, respectively (all P < 0.001). Mean medicated baseline IOP was reduced from 21.4 (3.6) to 14.9 (4.5) mmHg at 12 months and 15.2 (4.2) mmHg at 24 months, with similar results in both treatment groups. The clinical success rate was 67.6% at 12 months and 65.8% at 24 months. Overall, 51.1 (12 months) and 44.7% (24 months) of eyes were medication-free. The implant safety profile compared favorably with that published for trabeculectomy and tube shunts.
Conclusions
The gelatin implant effectively reduced IOP and medication needs over 2 years in POAG uncontrolled medically, with an acceptable safety profile.
ClinicalTrials.gov registration number: NCT02006693 (registered in the USA).
Similar content being viewed by others
Introduction
Topical therapy is the usual first-line treatment for open-angle glaucoma, but is often hampered by poor adherence [1, 2]. Traditional subconjunctival drainage procedures, such as trabeculectomy and tube shunts, lower IOP most effectively but are relatively invasive and associated with both short- and longer-term complications that may result in significant loss of visual acuity [3,4,5,6,7]. Newer minimally invasive glaucoma surgery (MIGS) that permits earlier intervention is becoming part of the treatment armamentarium for glaucoma, providing a better safety profile than conventional approaches [8]. MIGS devices that can be implanted in conjunction with cataract surgery to facilitate aqueous drainage into Schlemm’s canal [9] or the supraciliary space [10] offer modest IOP lowering. An ab interno gelatin stent (XEN®45, Allergan plc, Dublin, Ireland) that also meets the criteria for MIGS [11] bypasses conventional outflow pathways that are known to be obstructed in primary open-angle glaucoma (POAG) by creating a connection between the anterior chamber and subconjunctival space [8], in a manner similar to the gold standard trabeculectomy. The device is implanted ab interno, either as a stand-alone procedure or in combination with cataract surgery, without conjunctival dissection. The hydrophilic gelatin implant swells and conforms to surrounding tissues, which helps maintain its position post-implantation.
Results from studies demonstrating the IOP-lowering performance and safety of the gelatin implant at 1 year across a spectrum of glaucoma patients have been published [12,13,14,15,16,17]. The present study was designed to evaluate, over 2 years in typical clinical settings, the effectiveness of the gelatin implant as primary surgical intervention in reducing IOP and the number of topical IOP-lowering medications in patients with POAG uncontrolled on topical therapy.
Methods
Study design
This prospective, non-randomized, open-label, multicenter clinical study (ClinicalTrials.gov identifier: NCT02006693) was conducted between December 2013 and January 2017 in eight countries (Austria, Belgium, England, Germany, Italy, Poland, Spain, and Switzerland). The study complied with Good Clinical Practice/International Council for Harmonisation Guidelines, the Declaration of Helsinki, and all applicable country-specific regulations governing the conduct of clinical research, depending on which provided greater protection to the individual. The protocol was approved by an independent ethics committee prior to study start, and all patients were to provide written informed consent before initiating treatment.
Study population
The inclusion criteria were as follows: diagnosis of moderate POAG (defined by a mean deviation score between − 3 and − 12 dB) uncontrolled on topical therapy; medicated IOP ≥ 18 and ≤ 33 mmHg; use of one to four topical IOP-lowering medications; area of healthy, free, and mobile conjunctiva in the target quadrant; Shaffer angle grade ≥ 3 in the target quadrant; ≥ 18 years of age; signed written informed consent; and availability, willingness, and sufficient cognitive awareness to comply with the examination procedures and schedule.
Exclusion criteria included a diagnosis of any glaucoma other than POAG; prior incisional glaucoma surgery (prior iridotomy was acceptable if angles were open); prior cataract surgery in the study eye ≤ 3 months before study treatment; presence of scarring, prior surgery, or other pathologies in the conjunctiva (target quadrant); history of corneal surgery/disease; central corneal thickness ≤ 490 or ≥ 620 μm; presence of vitreous in the anterior chamber; presence of intraocular silicone oil; clinically significant inflammation or infection in the study eye within 30 days prior to the preoperative visit; active ophthalmic disease/disorder that could confound study results; impaired episcleral venous drainage; and known or suspected allergy/sensitivity to drugs required for the implantation (including anesthesia), or any of the device components (e.g., bovine or porcine products, and glutaraldehyde).
Both eyes could be implanted (study eyes) provided they met the eligibility criteria and surgeries for each eye were performed at least 30 days apart.
Perioperative procedures
The gelatin implant was placed ab interno either as a stand-alone procedure (implant alone) or in combination with cataract surgery (phaco + implant), based on whether the surgeon and patient deemed cataract surgery necessary at the time of glaucoma surgery.
Consistent with typical clinical practice, investigators could adjust the preoperative medication regimen as believed necessary/appropriate. Recommendations included a topical steroid (prednisolone acetate 1% or equivalent, or benzalkonium chloride [BAK]-free difluprednate 0.05%) four times daily (QID) in the study eye one week before surgery, and a topical antibiotic (fluoroquinolone or equivalent, preferably BAK-free) QID on day − 1 (preoperative). Topical (in the study eye) or systemic IOP-lowering medications were to be suspended on day 0 (surgery day). The surgery was performed using standard ophthalmic operating techniques and perioperative medications (including anesthesia), as customary for the investigator. Adjunctive antifibrotic therapy was administered pre-/perioperatively via subconjunctival injection, at the surgeon’s discretion (including type and dose).
In the implant alone group, an ab interno approach (described by Vera and Horvath [8]) was used to place the gelatin stent, connecting the anterior chamber to the subconjunctival space. General surgical steps for implantation included creating temporal clear corneal main and side port incisions; filling the anterior chamber with cohesive viscoelastic; inserting the needle tip of the injector through the main incision and advancing across the anterior chamber (toward the superior-nasal quadrant), with needle entry at the desired angle position and advancement through the sclera using a second instrument at the side port to provide stabilization and counterforce; visualizing the needle and needle tip bevel in the subconjunctival space; deploying the gelatin stent; removing the injector and viscoelastic; pressurizing the anterior chamber; and creating a subconjunctival bleb with a balanced salt solution. All incisions were hydrated at the end of the surgery. The target for an ideally positioned stent was 1 mm in the anterior chamber, 2 mm in the scleral tunnel, and 3 mm in the subconjunctival space. If incorrectly positioned, the device could be adjusted or exchanged.
In the phaco + implant group, phacoemulsification was performed and an intraocular lens was inserted, followed by placement of the gelatin implant if the cataract surgery was successful and uncomplicated. If complications that could potentially impact the study results (such as corneal burn, vitreous loss requiring vitrectomy, and placement of an anterior chamber lens) occurred during cataract surgery, the eye was withdrawn from the study.
The postoperative treatment regimen was per investigator’s discretion. Recommendations included topical antibiotic (fluoroquinolone or equivalent, preferably BAK-free) QID for 1 week, as well as topical steroid (prednisolone acetate 1% or equivalent, or BAK-free difluprednate 0.05%) QID for ≤ 4 weeks and titrated thereafter based on clinical assessment of postoperative inflammation. If a patient required further IOP lowering postoperatively, the investigator had the option of reintroducing ocular hypotensive medications in a step-wise fashion (i.e., 1 drug class at a time) and/or needling the bleb. Consistent with the American Academy of Ophthalmology’s Preferred Practice Pattern Guidelines [1], needling was part of the standard postoperative care to improve aqueous flow and lower IOP based on the investigator’s clinical assessment of bleb function. Consistent with other recent studies [18, 19], needling was not considered an adverse event (AE) or glaucoma-related secondary surgical intervention (SSI) but was documented as a postoperative procedure; it could be performed at any point in the postoperative period, as believed necessary by the investigator. No specific protocol was mandated, and use of an antifibrotic agent at the time of needling was also left to the investigator’s discretion.
Assessments
Postoperative visits were scheduled at day 1, weeks 1 and 2, and months 1, 3, 6, 9, 12, 18, and 24. IOP was determined at medicated baseline and each postoperative visit using Goldmann applanation tonometry and a masked, two-person method [20]; two consecutive measurements were taken, followed by a third if the first two differed by ≥ 3 mmHg. The average or median IOP was used for analysis, depending on whether two or three measurements were taken, respectively. Use of topical IOP-lowering medications was assessed at baseline and all postoperative visits.
Safety assessments included intraoperative complications (day 0 only), monocular best-corrected visual acuity—measured in Snellen (at all postoperative visits except day 1) and converted into logMAR for analysis, slit-lamp biomicroscopy, and postoperative AEs (at each postoperative visit). AEs of interest, such as shallow anterior chamber with iridocorneal touch, choroidal effusion, macular edema, macular folds, corneal erosion, and corneal edema, were specifically assessed and documented. Ophthalmoscopy (cup/disc ratio), pachymetry (central corneal thickness), and visual field (mean deviation) were assessed at baseline, month 12, and month 24.
Outcomes and analyses
All effectiveness analyses were performed using the modified intent-to-treat (mITT) population (i.e., all enrolled eyes [with verified informed consent documentation] that received an implant and met the IOP and IOP-lowering medication count inclusion criteria). The primary effectiveness outcomes were the changes in mean IOP and mean number of topical IOP-lowering medications in the study eyes from baseline to month 12; these parameters were also assessed at all other postoperative visits up to 24 months (secondary effectiveness outcomes).
Clinical success was defined as achieving ≥ 20% IOP reduction on the same or fewer IOP-lowering medications at month 12 (or 24), compared with baseline, without glaucoma-related SSI (which did not include needling) or intention to be converted to another procedure during the study.
Other effectiveness outcomes included the mean IOP and mean IOP-lowering medication count (topical) at each study visit, as well as the proportion of eyes achieving specific target IOPs, proportion of topical medication-free eyes and their mean IOP, proportion of eyes requiring needling, along with the mean number of needling procedures per eye, number of eyes with 1, 2, 3, or > 3 needling procedures, overall needling rate, needling rate by site, and clinical success rate in needled eyes, at 12 and 24 months. The median needling rate was also calculated based on the month-24 needling rate for each site. AEs were summarized by counts and percentages, using the safety population (i.e., all eyes enrolled in the study that received the gelatin implant). Descriptive statistics were used to summarize all endpoints in the overall population, based on observed data (i.e., without imputation for missing data). Statistical testing was also performed to compare the changes from baseline in mean IOP and mean IOP-lowering medication count at months 12 and 24 between treatment groups. Because 19 patients had both eyes treated, a random effect model [21] was used to adjust for correlation between those eyes. Analysis was also performed using only one eye per patient (i.e., the first treated eye). In addition, the differences in needling procedures per eye between groups were analyzed using the modified Wilcoxon rank-sum test (adjusting for correlation [22]). All analyses were generated using the SAS® software version 9.3 (SAS Institute Inc., Cary, NC, USA).
Enrollment of up to 200 eyes was planned; due to unknown variability of the procedure’s effect on IOP, a formal calculation of sample size was not performed. The study was remotely monitored using a risk-based monitoring approach with one onsite visit at the end of the study. Although an interim analysis at 12 months was performed as planned and interim data cuts presented in scientific meetings (listed above), reported herein is the final analysis, performed after completion of the 24-month visit.
Results
Demographics, baseline characteristics, and surgical parameters
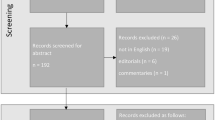
Overall, 240 eyes (217 patients) entered the study and 219 (200 patients) were enrolled (Fig. 1). A total of 218 eyes of 199 patients received the gelatin implant at 21 sites and were included in the safety population. The number (%) of patients implanted at each site ranged from 1 (0.5) to 28 (14.1) (mean 9.5; median 7.0). Overall, 197/218 (90.4%) eyes completed the 12-month visit; 174/218 (79.8%) completed the 24-month visit, while 44/218 (20.2%) discontinued the study (Fig. 1). No eye was withdrawn due to complications from cataract surgery. Data were comparable in the mITT population, with 182/202 (90.1%) and 161/202 (79.7%) eyes completing the 12- and 24-month visits, respectively, and 41/202 (20.3%) discontinuing the study. Patient demographics and baseline characteristics for the mITT population are summarized in Table 1; a total of 25 (23.6%) eyes in the implant alone group were pseudophakic.
Patient disposition. mITT, modified intent-to-treat; Phaco, phacoemulsification with intraocular lens placement. a Patients/eyes with verified informed consent documentation (incomplete consent documentation, N = 21). b Enrolled and received an implant (did not receive an implant, N = 1). c All enrolled eyes that received an implant and met the IOP and IOP-lowering medication count inclusion criteria. d No specific reasons were recorded
Adjunctive antifibrotic therapy (administered by subconjunctival injection) was used in all eyes; 99% received mitomycin C (MMC), while the remaining 1% received 5-fluorouracil (5-FU). The time of administration and absolute doses are detailed in Table 2.
Effectiveness
In the mITT population (N = 202), the change standard deviation (SD) in mean IOP from preoperative medicated baseline at 12 months (primary time point) was − 6.5 (5.3) mmHg (P < 0.001). The change (SD) in mean number of IOP-lowering medications was − 1.7 (1.3) (P < 0.001). Notably, the changes in both outcomes were also statistically significant at all other post-operative visits (P < 0.001), reaching − 6.2 (4.9) mmHg and − 1.5 (1.4) at month 24, respectively (Fig. 2). The mean percentage change in IOP from medicated baseline was − 29.3% at month 12 and − 27.8% at month 24 (Fig. 2).
Mean and changes in mean IOP and number of IOP-lowering medications from preoperative baseline over time in the mITT population (a) and implant alone vs. phaco + implant groups (b). IOP, intraocular pressure; meds, medications; mITT, modified intent-to-treat; phaco, phacoemulsification with intraocular lens replacement; preop, preoperative; SD, standard deviation. aP < 0.001 at all postoperative visits
Overall, results were similar in both treatment arms. The mean changes in IOP from medicated baseline were − 6.6 (5.6) and − 6.4 (5.0) mmHg at month 12 and − 6.4 (5.2) and − 5.9 (4.6) mmHg at month 24 in the implant alone and phaco + implant groups, respectively (P > 0.50). In these groups, the mean changes in IOP-lowering medication count were − 1.8 (1.3) and − 1.6 (1.2) at month 12 and − 1.5 (1.5) and − 1.5 (1.2) at month 24, respectively (P > 0.48). The mean percentage changes in IOP from medicated baseline were − 29.6 (month 12) and − 28.2% (month 24) in the former group and − 29.1 (month 12) and − 27.2% (month 24) in the latter (Fig. 2).
At 24 months, outcomes were also similar in pseudophakic eyes that received the implant alone (IOP reduction, − 8.4 mmHg; reduction in medication number, − 1.5; n = 15 in the mITT population) versus the overall implant alone group and the phaco + implant group. The outcomes also appeared similar between phakic (n = 80) and pseudophakic (n = 25) eyes that received the implant alone, although no statistical comparisons were made between these groups.
Because 19 patients had both eyes implanted, two sensitivity analyses were conducted. One used a random effect model to adjust for correlation between those eyes [21], while the other included only one eye per patient. Results of both sensitivity analyses were similar to the effectiveness outcomes described in the above paragraphs.
The clinical success rate was 67.6% at 12 months and 65.8% at 24 months in the mITT population (Figs. 3 and 4). Looking at specific IOP targets achieved at 12 and 24 months, 55.6 (n = 99/178) and 48.4% (n = 78/161) of eyes with available data achieved IOP reductions ≥ 30% from preoperative medicated baseline at 12 and 24 months, respectively (Fig. 5). In addition, the proportion of eyes achieving IOP ≤ 18, ≤ 15, and ≤ 12 mmHg was 83.7, 60.7, and 27.5% at 12 months, and 85.1, 62.7, and 24.2% at 24 months, respectively (Fig. 6).
Percentage of eyes with available data achieving success in the mITT population at months 12 and 24. Success was defined by eyes achieving ≥ 20% IOP reduction from baseline on the same or fewer medications without glaucoma-related secondary surgical intervention (SSI) or intention to be converted to another procedure. mITT, modified intent-to-treat; phaco, phacoemulsification with intraocular lens replacement
Kaplan-Meier curve showing the probability of achieving success criteria at months 12 and 24 (mITT population). Failures were due to glaucoma-related secondary surgical intervention (SSI—which did not include needling) during the study period, or IOP reduction < 20% on the same number of medications or fewer at 12 and 24 months. Right censoring assumption was used. Probability of success was 67% at month 12 and 58% at month 24. Note: eyes that did not undergo SSI but failed to meet success criteria at month 12 were deemed to have failed, even if they met the success criteria at month 24. Tick signs indicate censored times, corresponding to incidences when eyes discontinued early or completed the study without experiencing any failures. IOP, intraocular pressure; mITT, modified intent-to-treat
Scatter plots of IOP reduction as a function of preoperative IOP at month 12 (a) and month 24 (b) in the mITT population. Each data point represents one eye. The gray line marks the lower limit of medicated IOP required at baseline for eligibility (18 mmHg). The blue line delineates IOP reduction (lower portion) from IOP increase (upper portion), relative to baseline IOP. Data points below the 20, 30, and 40% IOP reduction lines achieved that level of IOP lowering or more. IOP, intraocular pressure; mITT, modified intent-to-treat. a Indicates IOP reduction success, as defined in the protocol
Remarkably, 51.1 (n = 91/178) and 44.7% (n = 72/161) of eyes with available data at 12 and 24 months were medication-free (topical), with a mean IOP (SD) of 13.8 (3.1) and 14.3 (3.1) mmHg, respectively (Table 3). Among the medication-free eyes, 79/91 (86.8%) and 59/72 (81.9%) achieved clinical success at 12 and 24 months, respectively.
Needling
In the mITT population, the overall needling rate was 41.1% (n = 83/202), without statistically significant differences between the implant alone (43.9%; n = 50/114 eyes) and phaco + implant (37.5%; n = 33/88) groups at month 24 (P > 0.5). The median needling rate was 33%.
The mean (SD) and median numbers of needling procedures per eye at 24 months were 1.6 (1.1) and 1.0 in the overall population, with a range of 1 to 6. The majority of needled eyes (n = 56/83; 67.5%) had one procedure (Table 4), and the mean time (SD) to the first needling was 152 (160) days (median, 90 days). In 74/83 (89.2%) cases, an antifibrotic agent (MMC, 5-FU, or other) was used during the procedure. The clinical success rate in needled eyes, as defined in the “Methods” section, was 59.2% at 12 months and 44.6% at 24 months.
Safety
Intraoperative complications were reported in 10 (4.6%) eyes, the most common being anterior chamber bleeding in 6 (2.8%) eyes (Table 5). A total of 65/218 (29.8%) eyes had one or more postoperative ocular AEs (Table 6); glaucoma-related SSI due to uncontrolled IOP (n = 14/218; 6.4%—the most common being trabeculectomy) and hyphema (n = 10/218; 4.6%) were most frequently reported (Table 6). Overall, 44/218 (20.2%) eyes had numeric hypotony (< 6 mmHg, self-resolved) within the first 2 postoperative weeks. Only 5/218, however, were recorded as AEs, all of which occurred within 1 week post-implantation and resolved by the 1-month visit without any intervention. No eyes had persistent hypotony, defined in the protocol as IOP < 6 mmHg at two consecutive postoperative visits > 30 days apart. Notably, there were no clinical hypotony-related complications (such as flat anterior chamber with iridocorneal touch extending to the pupil, hypotony maculopathy, and choroidal effusion lasting > 30 days [requiring surgical intervention]), retinal detachment, vitreous hemorrhage, or any other AE causing permanent visual impairment.
Three patients died during the study (due to decompensated cirrhosis, cardiac arrest, and hepatocellular carcinoma—not device/procedure-related), one of whom had both eyes implanted. Twelve patients (14 eyes) experienced non-fatal serious AEs (SAEs), of whom seven (nine eyes) had systemic SAEs. Six patients (six eyes) had ocular SAEs, five of which were in study eyes: cataract aggravated, retinal disorder (central retinal vein occlusion reported at 12 months, without elevated IOP), conjunctival erosion (implant exposure), glaucoma-related SSI (due to hospitalization for surgery), endophthalmitis (reported 15 months after implantation—detail provided in Table 6), and high IOP with SSI (cyclodestructive procedure) in the untreated fellow eye (n = 1 each; Table 6). One patient had both systemic and ocular SAEs.
Not unexpectedly, mean best-corrected visual acuity (SD, logMAR) improvements from baseline at 12 and 24 months were noted in the phaco + implant group (− 0.27 [0.24] and − 0.23 [0.24]), compared with the implant alone group (− 0.02 [0.19] and 0.01 [0.21]), respectively. Changes in mean central corneal thickness (SD) were not statistically significant at 12 and 24 months. The change from baseline in average visual field mean deviation was not statistically significant at 24 months (− 1.0 [8.3]; P = 0.138).
Discussion
This prospective, 24-month, non-randomized, open-label, multicenter study conducted in typical clinical settings assessed the long-term effectiveness and safety of the gelatin implant in patients with POAG uncontrolled on topical IOP-lowering medications. Mean IOP was reduced from 21.4 (3.6) (medicated baseline) to 14.9 (4.5) mmHg at month 12 and 15.2 (4.2) mmHg at month 24; the mean IOP-lowering medication count decreased from 2.7 (0.9) at baseline to 0.9 (1.1) at month 12 and 1.1 (1.2) at month 24. Similar results were observed in both treatment groups at all postoperative visits up to 24 months (P > 0.4, between-group comparisons). In addition, no differences in outcomes were noted at 24 months in pseudophakic eyes that received the implant alone, compared with the overall implant alone group and the phaco + implant group. These findings are consistent with other reports of studies with this device, including the US pivotal trial in refractory glaucoma [23], as well as independent, retrospective [14, 17, 18, 24,25,26] and prospective [12, 13, 15, 16, 19, 27,28,29] studies in glaucoma, showing effectiveness at 1 year. Among those, a prospective, open-label study of the implant used alone or in combination with cataract surgery (N = 149 eyes) [16] showed that the mean medicated IOP and mean number of medications decreased from 20.0 (7.1) mmHg and 1.9 (1.3) at baseline to 13.9 (4.3) mmHg (P < 0.01) and 0.5 (0.8) (P < 0.001) at 1 year, respectively. In our study, the mean percentage change in IOP from medicated baseline was − 29.3% at month 12, consistent with those published by Mansouri et al. (31% reduction) [16] and Grover et al. (35.6% reduction) [23], for example.
Although the patient populations and mode of administration of adjunctive antifibrotic therapy differed in the study by Grover et al. [23], the one by Mansouri et al. [16], and ours, the effectiveness of the gel stent in reducing IOP and need for IOP-lowering medications appear similar. In addition, our results not only demonstrate continued effectiveness of the gelatin implant at 2 years, with a mean % IOP reduction of 27.8%, but also show strikingly stable IOP values from month 1 to 2 years (despite a small, expected elevation at month 3 that may correlate with the median time to first needling). The clinical success rate also remained stable between months 12 (67.6%) and 24 (65.8%), further supporting the long-term effectiveness of the gelatin implant. Overall, 60.7 and 62.7% had IOP ≤ 15 mmHg at 12 and 24 months, respectively. It is also notable that the results were comparable whether implantation was performed as a stand-alone procedure or in combination with cataract surgery.
Needling can be an effective intervention in the postoperative management of gelatin stent implantation to restore bleb function, in line with recommendations by the American Academy of Ophthalmology after trabeculectomy [1]. There was variation in needling rate between study sites, as evidenced by the difference between the overall needling rate and the median needling rate. Overall, 41.1% of eyes underwent at least one needling procedure (74.7% [n = 62/83] occurring within the first 6 months post-surgery), and 44.6% of the needled eyes achieved clinical success criteria at month 24, with comparable results in both treatment groups.
The study results are also clinically relevant when compared with other MIGS devices. For instance, in a 2-year pivotal trial, no statistically significant difference in mean IOP reduction from a washed-out baseline was reported at 24 months between patients who received the trabecular micro-bypass stent during cataract surgery (mean IOP: 18.6 [3.4] mmHg at baseline, 17.1 [2.9] mmHg at 24 months) and those who underwent cataract surgery alone (mean IOP: 17.9 [3.0] mmHg at baseline, 17.8 [3.3] mmHg at 24 months) [30]. Similarly, the mean number of IOP-lowering medications used at 24 months was not statistically significantly different between treatment groups [30], suggesting limited long-term effectiveness of the device. We did not expect to see additional IOP lowering in the phaco + implant group, because many studies looking at trabeculectomy and phaco-trabeculectomy have shown comparable IOP lowering with both procedures [31,32,33,34,35,36,37,38]. Both phacoemulsification and trabeculectomy techniques have evolved, which might explain why more recent papers report no differences in outcomes between trabeculectomy alone vs combined with phacoemulsification. The gelatin stent relies on a similar outflow pathway as trabeculectomy [39,40,41], but is a much less invasive procedure and provides a more controlled outflow; these factors likely explain the lack of differences between the two groups observed in our study.
How the effectiveness of the trabecular micro-bypass stent compares with that of the gelatin implant remains to be determined because the primary and secondary outcomes in studies of the trabecular micro-bypass stent assessed IOP lowering from unmedicated/washed-out baseline [10, 30]. In our study, eyes did not undergo washout before surgery, so the baseline IOP was expectedly lower. Also, most patients included in this study had moderate POAG, with an average visual field mean deviation of − 8.0 dB, compared with − 3.9 dB in the trabecular micro-bypass study [10, 30].
In studies of trabeculectomy, the gold standard for filtering surgery in open-angle glaucoma, effective IOP lowering to low teens was reported, but this was associated with significant AEs. Although the Tube versus Trabeculectomy study did not report outcomes at 2 years, results at 1 [42] and 3 years [43] showed that 57 and 60% of patients in the trabeculectomy arm experienced postoperative complications, respectively, compared with 29.8% at 2 years in our study. In a retrospective study that evaluated the outcomes and risk factors for failure of the gelatin stent versus trabeculectomy [24], both procedures had a 75% survival of approximately 10 months without medications or additional surgery (complete success) and > 2 years with add-on medications or laser trabeculoplasty (qualified success). Notably, one quarter and one third of eyes treated with the gelatin stent and trabeculectomy, respectively, were receiving glaucoma medications at the last recorded visit [24].
In line with the increasing trend of subconjunctival injection of MMC in trabeculectomy, all eyes implanted in this study received subconjunctival antifibrotic injection (range: 10–80 μg for MMC; two patients received 500-μg 5-FU) to allow precise dosing, compared with the traditional sponge method [44, 45]. The study thus adds to the prospective data on the perioperative administration of MMC by subconjunctival injection with implantation of the gelatin stent, at dosages aligned with expert recommendations (10–40 μg) [46].
The device exhibited an acceptable safety profile. All cases of hypotony (defined as IOP < 6 mmHg) were self-limited and self-resolved within 1 month of surgery, similar to what was reported by Grover et al. [23]. Low IOP in the immediate post-implantation period seems less likely to lead to clinical hypotony-related complications, compared with similar IOP after trabeculectomy, and thus may be amenable to observation without immediate intervention [8, 47]. Although SAEs were rare during the 2-year study, the isolated case of endophthalmitis underscores the need for ongoing care and monitoring of patients following glaucoma filtering procedures, even when IOP is well controlled post-surgery.
Potential study limitations include some variability in the perioperative regimens, which may have impacted the study outcomes. Current recommendations from surgeons experienced with the gelatin stent suggest that preoperative preparation of the conjunctiva and ocular surface, placement closer to the 12 o’clock position, avoiding penetration of Schlemm’s canal during implantation, making sure that the implant is free and mobile under the conjunctiva at the end of surgery, and achieving specific target IOP on day 1 or a low week-1 delta IOP, among others, may help optimize outcomes; most, however, were not published and thus not implemented during this study [46]. At the time of initiation of this study, the gelatin stent was very new on the market and no best practices were established, so the study results also reflect the investigators’ learning curve with the surgery [46] and the variation in pre- and postoperative regimens associated with typical clinical settings. Another potential limitation is the fact that < 5% of the study population was of Asian and Black/African ethnicity (reported to have a higher risk of failure with trabeculectomy) [48,49,50]. Nevertheless, the results of this study demonstrate a favorable risk/benefit profile when compared with those published for more invasive surgeries like tube/trabeculectomy. Our findings are generalizable to eyes with POAG uncontrolled with topical hypotensive agents and provide evidence that can help clinical decision making.
As first surgical intervention, the gelatin implant was effective over 2 years in reducing both IOP and medication needs in patients with moderate POAG uncontrolled topically, with an acceptable safety profile. Used alone or in combination with cataract surgery, the gelatin implant lends itself to use earlier in the treatment paradigm, offering a minimally invasive surgical alternative for patients with target IOP in the mid-low teens who are uncontrolled on topical therapy or whose quality of life is low on topical polytherapy, as well as those who are non-adherent or intolerant to topical therapy.
References
American Academy of Ophthalmology (2015) Primary open-angle glaucoma—preferred practice pattern. http://www.aaojournal.org/article/S0161-6420(15)01276-2/pdf. Accessed January 23, 2018
European Glaucoma Society Terminology and guidelines for glaucoma (4th edition). https://www.eugs.org/eng/guidelines.asp. Accessed January 23, 2018
Lichter PR, Musch DC, Gillespie BW, Guire KE, Janz NK, Wren PA, Mills RP, CIGTS Study Group (2001) Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology 108:1943–1953. https://doi.org/10.1016/S0161-6420(01)00873-9
Feiner L, Piltz-Seymour JR (2003) Collaborative Initial Glaucoma Treatment Study: a summary of results to date. Curr Opin Ophthalmol 14:106–111
Jampel HD, Musch DC, Gillespie BW, Lichter PR, Wright MM, Guire KE (2005) Perioperative complications of trabeculectomy in the collaborative initial glaucoma treatment study (CIGTS). Am J Ophthalmol 140:16–22. https://doi.org/10.1016/j.ajo.2005.02.013
Zahid S, Musch DC, Niziol LM, Lichter PR (2013) Risk of endophthalmitis and other long-term complications of trabeculectomy in the Collaborative Initial Glaucoma Treatment Study (CIGTS). Am J Ophthalmol 155:674–680.e1. https://doi.org/10.1016/j.ajo.2012.10.017
Gedde SJ, Feuer WJ, Shi W, Lim KS, Barton K, Goyal S, Ahmed IIK, Brandt J (2018) Treatment outcomes in the primary tube versus trabeculectomy study after 1 year of follow-up. Ophthalmology 125:650–663. https://doi.org/10.1016/j.ophtha.2018.02.003
Vera VI, Horvath C (2014) XEN gel stent: the solution designed by AqueSys®. In: Samples JR, Ahmed IIK (eds) Surgical innovations in glaucoma. Springer Science+Business Media, New York, pp 189–198
Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE (2011) Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology 118:459–467. https://doi.org/10.1016/j.ophtha.2010.07.007
Vold S, Ahmed IIK, Craven ER, Mattox C, Stamper R, Packer M, Brown RH, Ianchulev T (2016) Two-year COMPASS trial results: supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology 123:2103–2112. https://doi.org/10.1016/j.ophtha.2016.06.032
Caprioli J, Kim JH, Friedman DS, Kiang T, Moster MR, Parrish RK 2nd, Rorer EM, Samuelson T, Tarver ME, Singh K, Eydelman MB (2015) Special commentary: supporting innovation for safe and effective minimally invasive glaucoma surgery: summary of a joint meeting of the American Glaucoma Society and the Food and Drug Administration, Washington, DC, February 26, 2014. Ophthalmology 122:1795–1801. https://doi.org/10.1016/j.ophtha.2015.02.029
De Gregorio A, Pedrotti E, Russo L, Morselli S (2017) Minimally invasive combined glaucoma and cataract surgery: clinical results of the smallest ab interno gel stent. Int Ophthalmol 38:1129–1134. https://doi.org/10.1007/s10792-017-0571-x
Galal A, Bilgic A, Eltanamly R, Osman A (2017) XEN glaucoma implant with mitomycin C 1-year follow-up: result and complications. J Ophthalmol 2017:5457246. https://doi.org/10.1155/2017/5457246
Hengerer FH, Kohnen T, Mueller M, Conrad-Hengerer I (2017) Ab interno gel implant for the treatment of glaucoma patients with or without prior glaucoma surgery: 1-year results. J Glaucoma 26:1130–1136. https://doi.org/10.1097/ijg.0000000000000803
Sng CC, Wang J, Hau S, Htoon HM, Barton K (2017) XEN-45 collagen implant for the treatment of uveitic glaucoma. Clin Exp Ophthalmol 46:339–34. https://doi.org/10.1111/ceo.13087
Mansouri K, Guidotti J, Rao HL, Ouabas A, D'Alessandro E, Roy S, Mermoud A (2018) Prospective evaluation of standalone XEN gel implant and combined phacoemulsification-XEN Gel implant surgery: 1-year results. J Glaucoma 27:140–147. https://doi.org/10.1097/IJG.0000000000000858
Tan SZ, Walkden A, Au L (2018) One-year result of XEN45 implant for glaucoma: efficacy, safety, and postoperative management. Eye 32:324–332. https://doi.org/10.1038/eye.2017.162
Ibáñez-Muñoz A, Soto-Biforcos VS, Chacón-González M, Rúa-Galisteo O, Arrieta-Los Santos A, Lizuain-Abadia ME, Del Río Mayor JL (2018) One-year follow-up of the XEN(R) implant with mitomycin-C in pseudoexfoliative glaucoma patients. Eur J Ophthalmol. https://doi.org/10.1177/1120672118795063
Mansouri K, Gillmann K, Rao HL, Guidotti J, Mermoud A (2018) Prospective evaluation of XEN gel implant in eyes with pseudoexfoliative glaucoma. J Glaucoma 27:869–873. https://doi.org/10.1097/ijg.0000000000001045
Parrish RK 2nd, Minckler DS, Lam D, Pfeiffer N, RojanaPongpun P (2009) Recommended methodology for glaucoma surgical trials. In: Shaarawy TM, Sherwood MB, Grehn F (eds) World Glaucoma Association Guidelines on design and reporting of glaucoma surgical trials. Kugler Publications, Amsterdam, pp 1–14
Armstrong RA (2013) Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol Opt 33:7–14. https://doi.org/10.1111/opo.12009
Rosner B, Glynn RJ, Lee ML (2003) Incorporation of clustering effects for the Wilcoxon rank sum test: a large-sample approach. Biometrics 59:1089–1098. https://doi.org/10.1111/j.0006-341X.2003.00125.x
Grover DS, Flynn WJ, Bashford KP, Lewis RA, Duh YJ, Nangia RS, Niksch B (2017) Performance and safety of a new ab interno gelatin stent in refractory glaucoma at 12 months. Am J Ophthalmol 183:25–36. https://doi.org/10.1016/j.ajo.2017.07.023
Schlenker MB, Gulamhusein H, Conrad-Hengerer I, Somers A, Lenzhofer M, Stalmans I, Reitsamer H, Hengerer FH, Ahmed IIK (2017) Efficacy, safety, and risk factors for failure of standalone ab interno gelatin microstent implantation versus standalone trabeculectomy. Ophthalmology 124:1579–1588. https://doi.org/10.1016/j.ophtha.2017.05.004
Ozal SA, Kaplaner O, Basar BB, Guclu H, Ozal E (2017) An innovation in glaucoma surgery: XEN45 gel stent implantation. Arq Bras Oftalmol 80:382–385. https://doi.org/10.5935/0004-2749.20170093
Widder RA, Dietlein TS, Dinslage S, Kuhnrich P, Rennings C, Rossler G (2018) The XEN45 Gel Stent as a minimally invasive procedure in glaucoma surgery: success rates, risk profile, and rates of re-surgery after 261 surgeries. Graefes Arch Clin Exp Ophthalmol 256:765–771. https://doi.org/10.1007/s00417-018-3899-7
Pérez-Torregrosa VT, Olate-Pérez A, Cerdà-Ibáñez M, Gargallo-Benedicto A, Osorio-Alayo V, Barreiro-Rego A, Duch-Samper A (2016) Combined phacoemulsification and XEN45 surgery from a temporal approach and 2 incisions. Arch Soc Esp Oftalmol 91:415–421. https://doi.org/10.1016/j.oftal.2016.02.006
Fea AM, Spinetta R, Cannizzo PML, Consolandi G, Lavia C, Aragno V, Germinetti F, Rolle T (2017) Evaluation of bleb morphology and reduction in IOP and glaucoma medication following implantation of a novel gel stent. J Ophthalmol 2017:9364910. https://doi.org/10.1155/2017/9364910
Hohberger B, Welge-Lüßen UC, Lämmer R (2018) MIGS: therapeutic success of combined Xen Gel Stent implantation with cataract surgery. Graefes Arch Clin Exp Ophthalmol 256:621–625. https://doi.org/10.1007/s00417-017-3895-3
Craven ER, Katz LJ, Wells JM, Giamporcaro JE (2012) Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg 38:1339–1345. https://doi.org/10.1016/j.jcrs.2012.03.025
Song BJ, Ramanathan M, Morales E, Law SK, Giaconi JA, Coleman AL, Caprioli J (2016) Trabeculectomy and combined phacoemulsification-trabeculectomy: outcomes and risk factors for failure in primary angle closure glaucoma. J Glaucoma 25:763–769. https://doi.org/10.1097/ijg.0000000000000493
Jung LJ, Isida-Llerandi CG, Lazcano-Gomez G, SooHoo JR, Kahook MY (2014) Intraocular pressure control after trabeculectomy, phacotrabeculectomy and phacoemulsification in a Hispanic population. J Curr Glaucoma Pract 8:67–74. https://doi.org/10.5005/jp-journals-10008-1164
Murthy SK, Damji KF, Pan Y, Hodge WG (2006) Trabeculectomy and phacotrabeculectomy, with mitomycin-C, show similar two-year target IOP outcomes. Can J Ophthalmol 41:51–59. https://doi.org/10.1016/s0008-4182(06)80067-0
Cillino S, Di Pace F, Casuccio A, Calvaruso L, Morreale D, Vadala M, Lodato G (2004) Deep sclerectomy versus punch trabeculectomy with or without phacoemulsification: a randomized clinical trial. J Glaucoma 13:500–506
Kuroda S, Mizoguchi T, Terauchi H, Nagata M (2001) Trabeculectomy combined with phacoemulsification and intraocular lens implantation. Semin Ophthalmol 16:168–171. https://doi.org/10.1076/soph.16.3.168.4203
Guggenbach M, Mojon DS, Böhnke M (1999) Evaluation of phacotrabeculectomy versus trabeculectomy alone. Ophthalmologica 213:367–370. https://doi.org/10.1159/000027456
Derick RJ, Evans J, Baker ND (1998) Combined phacoemulsification and trabeculectomy versus trabeculectomy alone: a comparison study using mitomycin-C. Ophthalmic Surg Lasers 29:707–713
Yu CB, Chong NH, Caesar RH, Boodhoo MG, Condon RW (1996) Long-term results of combined cataract and glaucoma surgery versus trabeculectomy alone in low-risk patients. J Cataract Refract Surg 22:352–357
Chang TC, Budenz DL, Liu A, Kim WI, Dang T, Li C, Iwach AG, Radhakrishnan S, Singh K (2012) Long-term effect of phacoemulsification on intraocular pressure using phakic fellow eye as control. J Cataract Refract Surg 38:866–870. https://doi.org/10.1016/j.jcrs.2012.01.016
Mansberger SL, Gordon MO, Jampel H, Bhorade A, Brandt JD, Wilson B, Kass MA (2012) Reduction in intraocular pressure after cataract extraction: the Ocular Hypertension Treatment Study. Ophthalmology 119:1826–1831. https://doi.org/10.1016/j.ophtha.2012.02.050
Slabaugh MA, Bojikian KD, Moore DB, Chen PP (2014) The effect of phacoemulsification on intraocular pressure in medically controlled open-angle glaucoma patients. Am J Ophthalmol 157:26–31. https://doi.org/10.1016/j.ajo.2013.08.023
Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC (2007) Surgical complications in the Tube Versus Trabeculectomy Study during the first year of follow-up. Am J Ophthalmol 143:23–31. https://doi.org/10.1016/j.ajo.2006.07.022
Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL (2009) Three-year follow-up of the Tube Versus Trabeculectomy Study. Am J Ophthalmol 148:670–684. https://doi.org/10.1016/j.ajo.2009.06.018
Khouri SA, Huang G, Huang LY (2017) Intraoperative injection vs sponge-applied mitomycin C during trabeculectomy: one-year study. J Curr Glaucoma Pract 11:101–106. https://doi.org/10.5005/jp-journals-10028-1233
Pakravan M, Esfandiari H, Yazdani S, Douzandeh A, Amouhashemi N, Yaseri M, Pakravan P (2017) Mitomycin C-augmented trabeculectomy: subtenon injection versus soaked sponges: a randomised clinical trial. Br J Ophthalmol 101:1275–1280. https://doi.org/10.1136/bjophthalmol-2016-309671
Vera V, Ahmed IIK, Stalmans I, Reitsamer H (2018) Gel stent implantation—recommendations for preoperative assessment, surgical technique, and postoperative management. US Ophthalmic Rev 11:38–46. https://doi.org/10.17925/USOR.2018.11.1.38
Vijaya L, Manish P, Ronnie G, Shantha B (2011) Management of complications in glaucoma surgery. Indian J Ophthalmol 59(Suppl):S131–S140. https://doi.org/10.4103/0301-4738.73689
Nguyen AH, Fatehi N, Romero P, Miraftabi A, Kim E, Morales E, Giaconi J, Coleman AL, Law SK, Caprioli J, Nouri-Mahdavi K (2018) Observational outcomes of initial trabeculectomy with mitomycin c in patients of African descent vs patients of European descent: five-year results. JAMA Ophthalmol 136:1106–1113. https://doi.org/10.1001/jamaophthalmol.2018.2897
Tan C, Chew PT, Lum WL, Chee C (1996) Trabeculectomy—success rates in a Singapore hospital. Singap Med J 37:505–507
Wong JS, Yip L, Tan C, Chew P (1998) Trabeculectomy survival with and without intra-operative 5-fluorouracil application in an Asian population. Aust N Z J Ophthalmol 26:283–288. https://doi.org/10.1111/j.1442-9071.1998.tb01331.x
Acknowledgments
The authors would like to recognize the contributions of Zhanying Bai, M.S. and Charlie Wu, M.S. (employees of Allergan) for their support with the statistical analyses and programming, Andrew Shirlaw, M.S. (employee of Allergan) for his contribution to study project management, as well as Mini Balaram, M.D. (employee of Allergan) for her contribution to the manuscript development.
Writing/editorial assistance was provided to the authors by Michele Jacob, Ph.D., CMPP, of Evidence Scientific Solutions, Inc. (Philadelphia, Pennsylvania) and funded by Allergan plc (Dublin, Ireland). All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship.
APEX Study Group
Ejaz Ansari, M.D. (Maidstone Hospital, Eye, Ear, and Mouth Unit, Maidstone Kent, England). Leon Au, M.D. (Department of Eye Research, Manchester Royal Eye Hospital, Manchester, England). Keith Barton, M.D., CP FCRS, FRCOphth (Moorfields Eye Hospital, London, England). H. Burkhard Dick, M.D., Ph.D., FEBOS-CR (University Eye Clinic Bochum, Bochum, Germany). Luis Cadarso, M.D. (Ophthalmology Department, Clínica Oftalmológica Dr. Cadarso, Pontevedra, Spain). Antonio Fea, M.D. (Instituto di Fisiopatologia Clinica, Clinica Oculistica, Universita’ di Torino, Torino, Italy). Fritz Hengerer, M.D. (Klinik für Augenheilkunde, Frankfurt, Germany). Helmut Höh, M.D., FEBO (Department of Ophthalmology, Dietrich-Bonhoeffer-Klinikum, Neubrandenburg, Germany). Cosme Lavin-Dapena, M.D. (Hospital Universitario La Paz, Madrid, Spain). Kin Sheng Lim, MBChB, M.D., FRCOphth (Ophthalmology Department, St Thomas’ Hospital, London, England). Giorgio Marchini, M.D. (University Eye Clinic, Department of Neurological and Movement Sciences, University of Verona, Verona, Italy). Imran Masood, M.D. (Birmingham Midland Eye Theaters, West Midlands, England). Georg Mossböck, M.D. (Medical University Graz, Graz, Austria). Madhu Nagar, M.D. (Clinical Research Team, Pinderfields Hospital, Wakefield, England). Marco Nardi, M.D. (University of Pisa, Pisa, Italy). Herbert Reitsamer, M.D. (Department of Ophthalmology and Optometry, University Clinic Salzburg, SALK/Paracelsus Medical University, Salzburg, Austria). Marek Rękas, M.D., Ph.D. (Ophthalmology Department of the Military Health Service Institute, Warsaw, Poland). Tarek Shaarawy, M.D. (University of Geneva, Geneva, Switzerland). Ingeborg Stalmans, M.D., Ph.D. (Department of Ophthalmology, University Hospitals UZ Leuven, Leuven, Belgium). Miguel Teus, M.D. (Hospital Universitario Principe de Asturias, Madrid, Spain). Clemens Vass, M.D. (Vienna University, Vienna, Austria).
Funding
This study was funded by Allergan plc (Dublin, Ireland; formerly AqueSys, Inc.).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Herbert Reitsamer has received consulting honoraria from Allergan. Chelvin Sng has received consulting honoraria from Allergan, Glaukos (San Clemente, California), Alcon (Fort Worth, Texas), and Santen Pharmaceuticals (Osaka, Japan). Vanessa Vera has received consulting honoraria from Allergan. Keith Barton has received consulting honoraria from Alcon, Allergan, and Glaukos. Ingeborg Stalmans has received consulting honoraria from Allergan. Markus Lenzhofer declares that he has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Reitsamer, H., Sng, C., Vera, V. et al. Two-year results of a multicenter study of the ab interno gelatin implant in medically uncontrolled primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol 257, 983–996 (2019). https://doi.org/10.1007/s00417-019-04251-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04251-z