Abstract
Background
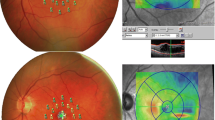
Macular contraction after anti-vascular endothelial growth factor (anti-VEGF) injections for diabetic macular edema (DME) was evaluated by documenting the displacement of macular capillary vessels and epiretinal membrane (ERM) formation.
Methods
A total of 130 eyes were included in this retrospective study. The study group consisted of 63 eyes which had intravitreal anti-VEGF injections for DME, and the control group included 67 eyes without central DME. The study and the control groups were well balanced in terms of diabetes duration and HbA1c. The distances between the bifurcation of the macular capillary retinal vessels were measured, and ERM status was evaluated based on spectral-OCT findings on the initial and final visit.
Results
In the study group, the mean number of injections was 4.7 ± 2.6 (3–14). The mean follow-up time was 16.7 ± 7.8 months in the study group whereas it was 20.7 ± 10.9 months in the control group (p = 0.132). The change in distance measurements between the reference points on macular capillary vessels was significant in all lines except line c (p < 0.05 for lines a, b, d, e, and f) in the study group whereas it was significant in only line e in the control group (p = 0.007, paired samples test). However, when the change in macular thickness was accounted as a confounding factor, the change in distances between the references points from the initial visit to the final visit lost its significance (repeated measures ANCOVA, p > 0.05). During follow-up, the number of cases with ERM changed from 10 to 12 in the study group whereas it remained three in the control group.
Conclusion
There was a displacement of macular capillary vessels which was associated with the change in macular thickness in eyes having anti-VEGF injections for DME. The number of ERM cases did not change significantly during the follow-up.

Similar content being viewed by others
References
Thomas BJ, Shienbaum G, Boyer DS et al (2003) Evolving strategies in the management of diabetic macular edema: clinical trials and current management. Can J Ophthalmol 48(1):22–30
Krispel C, Rodrigues M, Xin X et al (2013) Ranibizumab in diabetic macular edema. World J Diabetes 4(6):310–318
Wykoff CC, Hariprasad SM (2015) Comparing aflibercept, bevacizumab, and ranibizumab for DME: analysis of DRCR protocol T. Ophthalmic Surg Lasers Imaging Retina 46(3):302–305
Wells JA, Glassman AR, Jampol LM et al (2016) Diabetic retinopathy clinical research network. Association of baseline visual acuity and retinal thickness with 1-year efficacy of aflibercept, bevacizumab, and ranibizumab for diabetic macular edema. JAMA Ophthalmol 134(2):127–134
Nguyen QD, Shah SM, Khwaja AA et al (2010) READ-2 study group. Two-year outcomes of the Ranibizumab for Edema of the mAcula in Diabetes (READ-2) study. Ophthalmology 117:2146–2151
Massin P, Bandello F, Garweg JG et al (2010) Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care 33:2399–2405
Bu SC, Kuijer R, Li XR et al (2014) Idiopathic epiretinal membrane. Retina 34(12):2317–2335
Ophir A, Martinez MR (2011) Epiretinal membranes and incomplete posterior vitreous detachment in diabetic macular edema, detected by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 52(9):6414–6420
Ophir A, Martinez MR, Mosqueda P et al (2010) Vitreous traction and epiretinal membranes in diabetic macular oedema using spectral-domain optical coherencetomography. Eye (Lond) 24(10):1545–1553
Yamamoto T, Akabane N, Takeuchi S (2001) Vitrectomy for diabetic macular edema: the role of posterior vitreous detachment and epimacular membrane. Am J Ophthalmol 132(3):369–377
Ghazi NG, Ciralsky JB, Shah SM et al (2007) Optical coherence tomography findings in persistent diabetic macular edema: the vitreomacular interface. Am J Ophthalmol 144(5):747–754
Bressler SB, Qin H, Beck RW et al (2012) Diabetic retinopathy clinical research network. Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol 130(9):1153–1161
Yoon D, Rusu I, Barbazetto I (2014) Reduced effect of anti-vascular endothelial growth factor agents on diabetics with vitreomacular interface abnormalities. Int Ophthalmol 34(4):817–823
Zhang M, Chu S, Zeng F et al (2015) Bevacizumab modulates the process of fibrosis in vitro. Clin Exp Ophthalmol 43(2):173–179
Ramasubramanian A, Shields CL (2012) Bevacizumab for Coats’ disease with exudative retinal detachment and risk of vitreoretinal traction. Br J Ophthalmol 96(3):356–359
Osaadon P, Fagan XJ, Lifshitz T et al (2014) A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye (Lond) 28(5):510–520
Kuiper EJ, Van Nieuwenhoven FA, de Smet MD et al (2008) The angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathy. PLoS One 3(7):e2675
Kim JW, Choi KS (2014) Quantitative analysis of macular contraction in idiopathic epiretinal membrane. BMC Ophthalmol 14:51
Weinberger D, Stiebel-Kalish H, Priel E et al (1999) Digital red-free photography for the evaluation of retinal blood vessel displacement in epiretinal membrane. Ophthalmology 106(7):1380–1383
Kawano K, Ito Y, Kondo M et al (2013) Displacement of foveal area toward optic disc after macular hole surgery with internal limiting membrane peeling. Eye (Lond) 27(7):871–877
Wong Y, Steel DHW, Habib MS, Stubbing-Moore A, Bajwa D, Avery PJ (2017) Sunderland eye infirmary study group. Vitreoretinal interface abnormalities in patients treatedwith ranibizumab for diabetic macular oedema. Graefes Arch Clin Exp Ophthalmol 55(4):733–742
Chang CK, Cheng CK, Peng CH (2017) The incidence and risk factors for the development of vitreomacular interface abnormality in diabetic macular edema treated with intravitreal injection of anti-VEGF. Eye (Lond) 31(5):762–770
Torres-Soriano ME, Reyna-Castelán E, Hernández-Rojas M et al (2009) Tractional retinal detachment after intravitreal injection of bevacizumab in proliferative diabetic retinopathy. Retin Cases Brief Rep 3(1):70–73
Arevalo JF, Maia M, Flynn HW Jr et al (2008) Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol 92:213–216
Oshima Y, Shima C, Wakabayashi T et al (2009) Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology 116:927–938
Leask A, Abraham DJ (2004) TGF-beta signaling and the fibrotic response. FASEB J 18(7):816–827
Kita T, Hata Y, Kano K et al (2007) Transforming growth factor-beta2 and connective tissue growth factor in proliferative vitreoretinal diseases: possible involvement of hyalocytes and therapeutic potential of rho kinase inhibitor. Diabetes 56(1):231–238
Kuiper EJ, de Smet MD, van Meurs JC et al (2006) Association of connective tissue growth factor with fibrosis in vitreoretinal disorders in the human eye. Arch Ophthalmol 124(10):1457–1462
Goldschmeding R, Aten J, Ito Y et al (2000) Connective tissue growth factor: just another factor in renal fibrosis. Nephrol Dial Transplant 15(3):296–299
Perbal B (2004) CCN proteins: multifunctional signaling regulators. Lancet 363(9402):62–64
van Nieuwenhoven FA, Jensen LJ, Flyvbjerg A et al (2005) Imbalance of growth factor signaling in diabetic kidney disease: is connective tissue growth factor (CTGF, CCN2) the perfect intervention point. Nephrol Dial Transplant 20(1):6–10
Kabanarou SA, Xirou T, Mangouritsas G et al (2017) Full-thickness macular hole formation following anti-VEGF injections for neovascular age-related macular degeneration. Clin Interv Aging 26(12):911–915
Querques G, Souied EH, Soubrane G (2009) Macular hole following intravitreal ranibizumab injection for choroidal neovascular membrane caused by age-related macular degeneration. Acta Ophthalmol 87(2):235–237
Erdurman FC, Pellumbi A, Durukan AH (2012) Lamellar macular hole formation in a patient with diabetic CME treated by intravitreal bevacizumab injections. Ophthalmic Surg Lasers Imaging 30(43 Online):e87–e89
Veloso CE, Kanadani TM, Pereira FB et al (2015) Vitreomacular Interface after anti-vascular endothelial growth factor injections in Neovascular age-related VMacular degeneration. Ophthalmology 122(8):1569–1572
Geck U, Pustolla N, Baraki H et al (2013) Posterior vitreous detachment following intravitreal drug injection. Graefes Arch Clin Exp Ophthalmol 251(7):1691–1695
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Cetin, E.N., Demirtaş, Ö., Özbakış, N.C. et al. Quantitative assessment of macular contraction and vitreoretinal interface alterations in diabetic macular edema treated with intravitreal anti-VEGF injections. Graefes Arch Clin Exp Ophthalmol 256, 1801–1806 (2018). https://doi.org/10.1007/s00417-018-4042-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-018-4042-5