Abstract
Purpose
To evaluate a modified treat-and-extend (TAE) regimen of intravitreal aflibercept injection (IAI) for treatment-naïve patients with neovascular age-related macular degeneration (AMD).
Methods
Thirty-six eyes (36 patients) treated with the modified TAE regimen were evaluated at 12 months retrospectively. The modified TAE regimen consisted of three steps: 1) an induction phase, during which patients were treated with ≥ 3-monthly IAIs until exudative activity disappeared, 2) an observation phase, during which patients were monitored until exudative activity appeared, and 3) a TAE phase, for which the initial treatment interval was determined based on the disease recurrence interval, followed by treatment intervals changing by 2 weeks.
Results
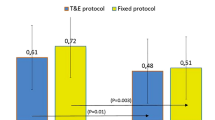
Mean logMAR BCVA improved significantly from 0.48 ± 0.51 at baseline to 0.40 ± 0.53 at 12 months (P < 0.01), and was maintained (losing <0.3 logMAR units) in 35 eyes (97.2 %). Mean central retinal thickness and central choroidal thickness decreased significantly after 12 months. In the TAE phase, the distribution of treatment intervals was ≥8 weeks in 64.7 % (11 eyes) at 12 months. The mean number of injections was 4.53.
Conclusion
A modified TAE regimen of IAI for neovascular AMD produced good functional outcomes over 12 months with the small number of injections.






Similar content being viewed by others
References
Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP (2004) Global data on visual impairment in the year 2002. Bull World Health Organ 82:844–851
Congdon N, O’Colmain B, Klaver CC, Klein R, Muñoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P, Eye Diseases Prevalence Research Group (2004) Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 122:477–485
Augustin AJ, Kirchhof J (2009) Inflammation and the pathogenesis of age-related macular degeneration. Expert Opin Ther Targets 13:641–651
Singerman LJ, Masonson H, Patel M, Adamis AP, Buggage R, Cunningham E, Goldbaum M, Katz B, Guyer D (2008) Pegaptanib sodium for neovascular age-related macular degeneration: third-year safety results of the VEGF Inhibition Study in Ocular Neovascularisation (VISION) trial. Br J Ophthalmol 92:1606–1611
Chakravarthy U, Adamis AP, Cunningham ET Jr, Goldbaum M, Guyer DR, Katz B, Patel M, VEGF Inhibition Study in Ocular Neovascularization (V.I.S.I.O.N.) Clinical Trial Group (2006) Year 2 efficacy results of 2 randomized controlled clinical trials of pegaptanib for neovascular age-related macular degeneration. Ophthalmology 113:1508–1521
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, MARINA Study Group (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355:1419–1431
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T, ANCHOR Study Group (2009) Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology 116:57–65
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD, Ho AC, Ogura Y, Simader C, Jaffe GJ, Slakter JS, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Sowade O, Zeitz O, Norenberg C, Sandbrink R, Heier JS (2014) Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology 121:193–201
Yonekawa Y, Andreoli C, Miller JB, Loewenstein JI, Sobrin L, Eliott D, Vavvas DG, Miller JW, Kim IK (2013) Conversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degeneration. Am J Ophthalmol 156:29–35
Cho H, Shah CP, Weber M, Heier JS (2013) Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol 97:1032–1035
Grewal DS, Gill MK, Sarezky D, Lyon AT, Mirza RG (2014) Visual and anatomical outcomes following intravitreal aflibercept in eyes with recalcitrant neovascular age-related macular degeneration: 12-month results. Eye (Lond) 28:895–899
Kawashima Y, Oishi A, Tsujikawa A, Yamashiro K, Miyake M, Ueda-Arakawa N, Yoshikawa M, Takahashi A, Yoshimura N (2015) Effects of aflibercept for ranibizumab-resistant neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol 253:1471–1477
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Groetzbach G, Sommerauer B, Sandbrink R, Simader C, Schmidt-Erfurth U, VIEW 1 and VIEW 2 Study Groups (2012) Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 119:2537–2548
Abedi F, Wickremasinghe S, Islam AF, Inglis KM, Guymer RH (2014) Anti-VEGF treatment in neovascular age-related macular degeneration: a treat-and-extend protocol over 2 years. Retina 34:1531–1538
Berg K, Pedersen TR, Sandvik L, Bragadóttir R (2015) Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology 122:146–152
Mantel I, Niderprim SA, Gianniou C, Deli A, Ambresin A (2014) Reducing the clinical burden of ranibizumab treatment for neovascular age-related macular degeneration using an individually planned regimen. Br J Ophthalmol 98:1192–1196
Holz FG, Amoaku W, Donate J, Guymer RH, Kellner U, Schlingemann RO, Weichselberger A, Staurenghi G, SUSTAIN Study Group (2011) Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology 118:663–671
Kuroda Y, Yamashiro K, Miyake M, Yoshikawa M, Nakanishi H, Oishi A, Tamura H, Ooto S, Tsujikawa A, Yoshimura N (2015) Factors associated with recurrence of age-related macular degeneration after anti-vascular endothelial growth factor treatment: A retrospective cohort study. Ophthalmology 122:2303–2310
Rayess N, Houston SK III, Gupta OP, Ho AC, Regillo CD (2015) Treatment outcomes after 3 years in neovascular age-related macular degeneration using a treat-and-extend regimen. Am J Ophthalmol 159:3–8
McKibbin M, Devonport H, Gale R, Gavin M, Lotery A, Mahmood S, Patel PJ, Ross A, Sivaprasad S, Talks J, Walters G (2015) Aflibercept in wet AMD beyond the first year of treatment: recommendations by an expert roundtable panel. Eye (Lond) 29:S1–S11
Wolf A, Kampik A (2014) Efficacy of treatment with ranibizumab in patients with wet age-related macular degeneration in routine clinical care: data from the COMPASS health services research. Graefes Arch Clin Exp Ophthalmol 252:647–655
Wecker T, Ehlken C, Bühler A, Lange C, Agostini H, Böhringer D, Stahl A (2016) Five-year visual acuity outcomes and injection patterns in patients with pro-re-nata treatments for AMD, DME, RVO and myopic CNV. Br J Ophthalmol. doi:10.1136/bjophthalmol-2016-308668
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
For this type of study, formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Ohnaka, M., Nagai, Y., Sho, K. et al. A modified treat-and-extend regimen of aflibercept for treatment-naïve patients with neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 255, 657–664 (2017). https://doi.org/10.1007/s00417-016-3507-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-016-3507-7