Abstract
Purpose
To compare outcomes between an as-needed and a treat-and-extend regimen in managing diabetic macular edema with intravitreal ranibizumab.
Methods
This was a retrospective, single-centre, comparative case series on 46 treatment naive patients with diabetic macular edema. Twenty-two patients were treated following an optical coherence tomography guided treat-and-extend protocol (OCTER), and 24 patients were treated according to a visual acuity guided pro re nata regimen (VAPRN) at a tertiarry referral centre. The main outcome measures were best-corrected visual acuity, central retinal thickness, and the number of ranibizumab injections, as well as visits after 12 months of treatment.
Results
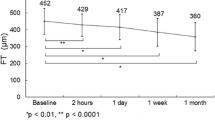
After 12 months, the mean gain in best-corrected visual acuity (± standard deviation) was 8.3 ± 6.7 versus 9.3 ± 8.9 letters in the VAPRN and OCTER group, respectively (p = 0.3). The mean decrease in central retinal thickness was 68.1 ± 88.0 μm in the VAPRN group and 117.6 ± 114.4 μm in the OCTER group (p = 0.2). The mean number of ranibizumab injections was significantly different between the VAPRN (5.9 ± 1.8) and the OCTER protocol (8.9 ± 2.0) (p < 0.001).
Conclusion
The visual acuity driven retreatment regimen resulted in a similar visual acuity outcome like optical coherence tomography guided retreatment for diabetic macular edema. Although the number of visits was similar in both groups, patients in the VAPRN group received significantly fewer intravitreal injections than patients in the OCTER group.



Similar content being viewed by others
References
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O’Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY, Meta-Analysis for Eye Disease Study G (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35:556–564. doi:10.2337/dc11-1909
Ferrara N, Damico L, Shams N, Lowman H, Kim R (2006) Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 26:859–870. doi:10.1097/01.iae.0000242842.14624.e7
Chen G, Li W, Tzekov R, Jiang F, Mao S, Tong Y (2014) Ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema: a meta-analysis of randomized controlled trials. PLoS One 9:e115797. doi:10.1371/journal.pone.0115797
Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, Gibson A, Sy J, Rundle AC, Hopkins JJ, Rubio RG, Ehrlich JS (2012) Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 119:789–801. doi:10.1016/j.ophtha.2011.12.039
Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, Sutter F, Simader C, Burian G, Gerstner O, Weichselberger A (2011) The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 118:615–625. doi:10.1016/j.ophtha.2011.01.031
Schmidt-Erfurth U, Lang GE, Holz FG, Schlingemann RO, Lanzetta P, Massin P, Gerstner O, Bouazza AS, Shen H, Osborne A, Mitchell P, Group RES (2014) Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology 121:1045–1053. doi:10.1016/j.ophtha.2013.11.041
Freund KB, Korobelnik JF, Devenyi R, Framme C, Galic J, Herbert E, Hoerauf H, Lanzetta P, Michels S, Mitchell P, Mones J, Regillo C, Tadayoni R, Talks J, Wolf S (2015) Treat-and-extend regimens with anti-vegf agents in retinal diseases: a literature review and consensus recommendations. Retina 35:1489–1506. doi:10.1097/iae.0000000000000627
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381. doi:10.1016/j.jbi.2008.08.010
Prünte C, Fajnkuchen F, Mahmood S, Ricci F, Hatz K, Studnička J, Bezlyak V, Parikh S, Stubbings WJ, Wenzel A, Figueira J (2016) Ranibizumab 0.5 mg treat-and-extend regimen for diabetic macular oedema: the RETAIN study. Br J Ophthalmol 100:787–795. doi:10.1136/bjophthalmol-2015-307249
Pearce I, Banerjee S, Burton BJ, Chakravarthy U, Downey L, Gale RP, Gibson J, Pagliarini S, Patel J, Sivaprasad S, Andrews C, Brittain C, Warburton J, RELIGHT Study Group (2015) Ranibizumab 0.5 mg for diabetic macular edema with bimonthly monitoring after a phase of initial treatment: 18-month, multicenter, phase IIIB RELIGHT study. Ophthalmology 122:1811–1819. doi:10.1016/j.ophtha.2015.05.038
Pervin LA (1963) The need to predict and control under conditions of threat. J Pers 31:570–587
Bredemeier K, Berenbaum H (2008) Intolerance of uncertainty and perceived threat. Behav Res Ther 46:28–38
Jin J, Sklar GE, Min Sen Oh V, Chuen Li S (2008) Factors affecting therapeutic compliance: a review from the patient’s perspective. Ther Clin Risk Manag 4:269–286
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research
Conflicts of interest
Andreas Ebneter has received speaker honorarium from Bayer, educational support from Novartis, and educational support from Allergan for which he also served as an advisor.
Dominik Waldmeier and Denise C Zysset-Burri do not have any conflict of interest.
Sebastian Wolf has served as a consultant for Allergan, Bayer, Heidelberg Engineering, Novartis, Optos Inc, Zeiss and Roche, and also received grant support from Heidelberg Engineering. Furthermore, he is a board member of Euretina.
Martin Zinkernagel has served as a consultant for Allergan, Bayer, Heidelberg Engineering, and Novartis in which he also owns stock. Furthermore, he receives research grants from Bayer and Heidelberg Engineering.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the local Ethics Committee (KEK-Nr. 093/13). For this type of retrospective study formal consent is not required.
Additional information
Andreas Ebneter and Dominik Waldmeier contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure
Number of ranibizumab injections in patients treated according to the visual acuity guided as-needed (VAPRN, red) regimen and the OCT guided treat-and-extend (OCTER, blue) protocol.Boxplot (A) of mean numbers of injections: Vertical bars are standard deviations (SD). P values from unpaired t-test.Density plot for injection likelihood (B): Graphical display of the likelihood estimate that a patient will be given intravitreal ranibizumab treatment at a specific time point after the start of the treatment depending on the treatment regimen. These kernel density plots were created based on the scatterplots shown in (C) and (D), using the function ‘density(x)’ from the software R (accessible at: https://www.r-project.org).Scatterplots representing single injections administered to individual patients in the VAPRN (red; C) and OCTER (blue; D) group over 12 months. Each dot represents an injectionVAPRN group: n = 24, OCTER group: n = 22.(JPG 2417 kb)
Rights and permissions
About this article
Cite this article
Ebneter, A., Waldmeier, D., Zysset-Burri, D.C. et al. Comparison of two individualized treatment regimens with ranibizumab for diabetic macular edema. Graefes Arch Clin Exp Ophthalmol 255, 549–555 (2017). https://doi.org/10.1007/s00417-016-3502-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-016-3502-z