Abstract
Purpose
To investigate structural changes in the retina by histologic evaluation and in vivo spectral domain optical coherence tomography (SD-OCT) following selective retina therapy (SRT) controlled by optical feedback techniques (OFT).
Methods
SRT was applied to 12 eyes of Dutch Belted rabbits. Retinal changes were assessed based on fundus photography, fluorescein angiography (FAG), SD-OCT, light microscopy, transmission electron microscopy (TEM), and scanning electron microscopy (SEM) at each of the following time points: 1 h, and 1, 3, 7, 14 and 28 days after SRT. BrdU (5’-bromo-2’-deoxy-uridine) incorporation assay was also conducted to evaluate potential proliferation of RPE cells.
Results
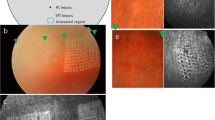
SRT lesions at1 h after SRT were ophthalmoscopically invisible. FAG showed leakage in areas corresponding to SRT lesions, and hyperfluorescence disappeared after 7 days. SD-OCT showed that decreased reflectivity corresponding to RPE damage was restored to normal over time in SRT lesions. Histologic analysis revealed that the damage in SRT lesions was primarily limited to the retinal pigment epithelium (RPE) and the outer segments of the photoreceptors. SEM and TEM showed RPE cell migration by day 3 after SRT, and restoration of the RPE monolayer with microvilli by 1 week after SRT. At 14 and 28 days, ultrastructures of the RPE, including the microvilli and tight junctions, were completely restored. The outer segments of the photoreceptors also recovered without sequelae. Interdigitation between the RPE and photoreceptors was observed. BrdU incorporation assay revealed proliferation of RPE on day 3 after SRT, and peak proliferation was observed on day 7 after SRT.
Conclusion
Based on multimodal imaging and histologic assessment, our findings demonstrate that SRT with OFT could selectively target the RPE without damaging the neurosensory retina. Therefore, the use of SRT with OFT opens the door to the possibility of clinical trials of well-defined invisible and nondestructive retina therapy, especially for macular disease.








Similar content being viewed by others
References
Early Treatment Diabetic Retinopathy Study Research Group (1987) Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early treatment diabetic retinopathy study report number 2. Ophthalmology 94:761–74
Gabel VP, Birngruber R, Hillenkamp F (1978) Visible and near infrared light absorption in pigment epithelium and choroid. In: Shimizu K (ed) International congress series No. 450, XXIII concilium ophthalmologicum, Kyoto. Excerpta Medicapp, Princeton, pp 658–62
Birngruber R, Hillenkamp F, Gabel VP (1985) Theoretical investigations of laser thermal retinal injury. Health Phys 48:781–796
Lorenz B, Birngruber R, Vogel A (1989) Quantifizierung der Wellenla¨ngenabha¨ngigkeitlaser induzierter Aderhauteffekte. Fortschr Ophthalmol 86:644–654
Marshall J, Mellerio J (1968) Pathological development of retinal laser photocoagulations. Exp Eye Res 7:225–230
Schatz H, Madeira D, McDonald HR, Johnson RN (1991) Progressive enlargement of laser scars following grid laser photocoagulation for diffuse diabetic macular edema. Arch Ophthalmol 109:1549–1551
Wallow IH, Birngruber R, Gabel VP, Hillenkamp F, Lund OE (1975) Netzhautreaktionnach Intensivlichtbestrahlung. Adv Ophthalmol 31:159–232
Pearson AR, Tanner V, Keightley SJ, Casswell AG (1998) What effect does laser photocoagulation have on driving visual fields in diabetics. Eye (Lond) 12:64–8
Ulbig MR, Arden GB, Hamilton AM (1994) Color contrast sensitivity and pattern electroretinographic findings after diode and argon laser photocoagulation in diabetic retinopathy. Am J Ophthalmol 117:583–8
Roider J, Hillenkamp F, Flotte T, Birngruber R (1993) Microphotocoagulation: selective effects of repetitive short laser pulses. Proc Natl Acad Sci U S A 90:8643–7
Bresnick GH (1983) Diabetic maculopathy: a critical review highlighting diffuse macular edema. Ophthalmology 90:1301–17
Brinkmann R, Roider J, Birngruber R (2006) Selective retina therapy (SRT): a review on methods, techniques, preclinical and first clinical results. Bull Soc Belge Ophtalmol 302:51–69
Elsner H, Pörksen E, Klatt C, Bunse A, Theisen-Kunde D, Brinkmann R, Birngruber R, Laqua H, Roider J (2006) Selective retina therapy in patients with central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol 244:1638–45
Roider J, Michaud NA, Flotte TJ, Birngruber R (1992) Response of the retinal pigment epithelium to selective photocoagulation. Arch Ophthalmol 110:1786–92
Laursen ML, Moeller F, Sander B, Sjoelie AK (2004) Subthreshold micropulse diode laser treatment in diabetic macular oedema. Br J Ophthalmol 88:1173–9
Parodi MB, Spasse S, Iacono P, Di Stefano G, Canziani T, Ravalico G (2006) Subthreshold grid laser treatment of macular edema secondary to branch retinal vein occlusion with micropulse infrared (810 nanometer) diode laser. Ophthalmology 113:2237–42
Roider J, Brinkmann R, Wirbelauer C, Laqua H, Birngruber R (2000) Subthreshold (retinal pigment epithelium) photocoagulation in macular diseases: a pilot study. Br J Ophthalmol 84:40–7
Yu AK, Merrill KD, Truong SN, Forward KM, Morse LS, Telander DG (2013) The comparative histologic effects of subthreshold 532- and 810-nm diode micropulse laser on the retina. Invest Ophthalmol Vis Sci 54:2216–24
Schmidt SY, Peisch RD (1986) Melanin concentration in normal human retinal pigment epithelium. Regional variation and age-related reduction. Invest Ophthalmol Vis Sci 27:1063–7
Brinkmann R, Koinzer S, Schlott K, Ptaszynski L, Bever M, Baade A, Luft S, Miura Y, Roider J, Birngruber R (2012) Real-time temperature determination during retinal photocoagulation on patients. J Biomed Opt 17:061219
Schlott K, Koinzer S, Ptaszynski L, Bever M, Baade A, Roider J, Birngruber R, Brinkmann R (2012) Automatic temperature controlled retinal photocoagulation. J Biomed Opt 17:061223
Park YG, Seifert E, Roh YJ, Theisen-Kunde D, Kang S, Brinkmann R (2014) Tissue response of selective retina therapy by means of a feedback-controlled energy ramping mode. Clin Experiment Ophthalmol 42:846–55
Kim HD, Han JW, Ohn YH, Brinkmann R, Park TK (2014) Functional evaluation using multifocal electroretinogram after selective retina therapy with a microsecond-pulsed laser. Invest Ophthalmol Vis Sci 56:122–31
Lopez PF, Yan Q, Kohen L, Rao NA, Spee C, Black J, Oganesian A (1995) Retinal pigment epithelial wound healing in vivo. Arch Ophthalmol 113:1437–46
Lavinsky D, Cardillo JA, Mandel Y, Huie P, Melo LA, Farah ME, Belfort R, Palanker D (2013) Restoration of retinal morphology and residual scarring after photocoagulation. Acta Ophthalmol 91:315–23
Oganesian A, Bueno E, Yan Q, Spee C, Black J, Rao NA, Lopez PF (1997) Scanning and transmission electron microscopic findings during RPE wound healing in vivo. Int Ophthalmol 21:165–75
Lavinsky D, Chalberg TW, Mandel Y, Huie P, Dalal R, Marmor M, Palanker D (2013) Modulation of transgene expression in retinal gene therapy by selective laser treatment. Invest Ophthalmol Vis Sci 54:1873–80
Paulus YM, Jain A, Gariano RF, Stanzel BV, Marmor M, Blumenkranz MS, Palanker D (2008) Healing of retinal photocoagulation lesions. Invest Ophthalmol Vis Sci 49:5540–5
Elner SG, Elner VM (1996) The integrin superfamily and the eye. Invest Ophthalmol Vis Sci 37:696–701
Rizzolo LJ (1991) Basement membrane stimulates the polarized distribution of integrins but not the Na, K-ATPase in the retinal pigment epithelium. Cell Regul 2:939–49
Rodriguez-Boulan E, Nelson WJ (1989) Morphogenesis of the polarized epithelial cell phenotype. Science 245:718–25
Prahs P, Walter A, Regler R, Theisen-Kunde D, Birngruber R, Brinkmann R, Framme C (2010) Selective retina therapy (SRT) in patients with geographic atrophy due to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 248:651–8
Framme C, Brinkmann R, Birngruber R, Roider J (2002) Autofluorescence imaging after selective RPE laser treatment in macular diseases and clinical outcome: a pilot study. Br J Ophthalmol 86:1099–106
Li R, Maminishkis A, Wang FE, Miller SS (2007) PDGF-C and D induced proliferation/migration of human RPE is abolished by inflammatory cytokines. Invest Ophthalmol Vis Sci 48:5722–32
Pons M, Marin-Castaño ME (2011) Nicotine increases the VEGF/PEDF ratio in retinal pigment epithelium: a possible mechanism for CNV in passive smokers with AMD. Invest Ophthalmol Vis Sci 52:3842–53
Sonoda S, Sreekumar PG, Kase S, Spee C, Ryan SJ, Kannan R, Hinton DR (2009) Attainment of polarity promotes growth factor secretion by retinal pigment epithelial cells: relevance to age-related macular degeneration. Aging (Albany NY) 2:28–42
Zhu J, Wang YS, Zhang J, Zhao W, Yang XM, Li X, Jiang TS, Yao LB (2009) Focal adhesion kinase signaling pathway participates in the formation of choroidal neovascularization and regulates the proliferation and migration of choroidal microvascular endothelial cells by acting through HIF-1 and VEGF expression in RPE cells. Exp Eye Res 88:910–8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in this study involving animals were in accordance with the ethical standards of the institution or practice at which the study was conducted.
Funding
The authors received no financial support for this research.
Conflict of interest
All authors involved in this study certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; or expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Ji Ho Yang and Seung-Young Yu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, J.H., Yu, SY., Kim, T.G. et al. Morphologic changes in the retina after selective retina therapy. Graefes Arch Clin Exp Ophthalmol 254, 1099–1109 (2016). https://doi.org/10.1007/s00417-016-3331-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-016-3331-0