Abstract
Background
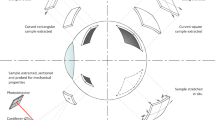
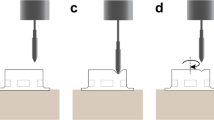
To illustrate the importance of biomechanical impact on tissue health within the central nervous system (CNS), we herein describe an in vitro model of rhegmatogenous retinal detachment (RRD) in which disruption and restoration of physical tissue support can be studied in isolation.
Methods
Adult retinal porcine explants were kept in culture for 3 or 12 hours without any tissue support, simulating clinical RRD, after which they were either maintained in this state or reattached to the culture membrane for an additional 48 hours.
Results
In vitro detachment resulted in gliosis and severe progressive loss of retinal neurons. In contrast, if the explant was reattached, gliosis and overall cell death was attenuated, ganglion cell death was arrested, and the number of transducin-expressing cone photoreceptors increased.
Conclusions
These results support the hypothesis that removal of the elastic retina from its normal physical environment results in degenerative damage, and, if restored, rescues retinal neurons. Our study reinforces the notion of a strong relationship between the biomechanical environment and homeostasis within the retina, which has significant bearing on pathologic events related to RRD, and may also have impact on other regions within the CNS under biomechanical influence.










Similar content being viewed by others
References
DuFort CC, Paszek MJ, Weaver VM (2011) Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol 12(5):308–319
Reichenbach A et al (1991) Development of the rabbit retina. IV. tissue tensility and elasticity in dependence on topographic specializations. Exp Eye Res 53(2):241–251
Schatz P, Holm K, Andreasson S (2007) Retinal function after scleral buckling for recent onset rhegmatogenous retinal detachment: assessment with electroretinography and optical coherence tomography. Retina 27:30–36
Schwartz SG, Flynn HW Jr, Mieler WF (2013) Update on retinal detachment surgery. Curr Opin Ophthalmol 24(3):255–261
Gong Y, Wu X, Sun X, Zhang X, Zhu P (2008) Electroretinogram changes after scleral buckling surgery of retinal detachment. Doc Ophthalmol 117(2):103–109
Algvere PV, Jahnberg P, Textorius O (1999) The Swedish retinal detachment register. I. a database for epidemiological and clinical studies. Graefes Arch Clin Exp Ophthalmol 237(2):137–144
Haimann MH, Burton TC, Brown CK (1982) Epidemiology of retinal detachment. Arch Ophthalmol 100(2):289–292
Johansson K, Malmsjö M, Ghosh F (2006) Tailored vitrectomy and laser photocoagulation without scleral buckling for primary rhegmatogenous retinal detachment. Br J Ophthalmol 90:1286–1291
Wang M, Munch IC, Hasler PW, Prünte C, Larsen M (2008) Central serous chorioretinopathy. Acta Ophthalmol 86(2):126–145
Sørensen NF et al (2012) The effect of subretinal viscoelastics on the porcine retinal function. Graefe’s Arch Clin Exp Ophthalmol 250:79–86
Taylor L, Moran D, Arnér K, Warrant E, Ghosh F (2013) Stretch to see—lateral tension strongly determines cell survival in long-term cultures of adult porcine retina. Invest Ophthalmol Vis Sci 54:1845–1856
Taylor L, Arnér K, Holmgren Taylor I, Ghosh F (2014) Feet on the ground: physical support of the inner retina is a strong determinant for cell survival and structural preservation in vitro. Invest Ophthalmol Vis Sci 55:2200–2213
Chandler MJ, Smith PJ, Samuelson DA, MacKay EO (1999) Photoreceptor density of the domestic pig retina. Vet Ophthalmol 2:179–184
García M, Ruiz-Ederra J, Hernández-Barbáchano H, Vecino E (2005) Topography of pig retinal ganglion cells. J Comp Neurol 486(4):361–372
Manouchehrian O, Arnér K, Deierborg T, Taylor L (2015) Who let the dogs out?: detrimental role of Galectin-3 in hypoperfusion-induced retinal degeneration. J Neuroinflammation 12:92
Ghosh F, Johansson K (2012) Neuronal and glial alterations in complex long-term rhegmatogenous retinal detachment. Curr Eye Res 37:704–711
Pastor JC et al (2006) Intraretinal immunohistochemistry findings in proliferative vitreoretinopathy with retinal shortening. Ophthalmic Res 38(4):193–200
Rex TS et al (2002) A survey of molecular expression by photoreceptors after experimental retinal detachment. Invest Ophthalmol Vis Sci 43(4):1234–1247
Fisher SK, Lewis GP, Linberg KA, Verardo MR (2005) Cellular remodeling in mammalian retina: results from studies of experimental retinal detachment. Prog Retin Eye Res 24:395–431
Matsumoto H et al (2014) Strain difference in photoreceptor cell death after retinal detachment in mice. Invest Ophthalmol Vis Sci 22 55(7):4165–4174
Iandiev I et al (2006) Glial cell reactivity in a porcine model of retinal detachment. Invest Ophthalmol Vis Sci 47:2161–2171
Lu YB et al (2006) Viscoelastic properties of individual glial cells and neurons in the CNS. Proc Natl Acad Sci U S A 103:17759–17764
Lu YB et al (2011) Reactive glial cells: increased stiffness correlates with increased intermediate filament expression. FASEB J 25:624–631
Ryskamp DA et al (2011) The polymodal ion channel transient receptor potential vanilloid 4 modulates calcium flux, spiking rate, and apoptosis of mouse retinal ganglion cells. J Neurosci 31(19):7089–7101
Bringmann A et al (2006) Müller cells in the healthy and diseased retina. Prog Retin Eye Res 25:397–424
Bringmann A et al (2009) Cellular signaling and factors involved in Müller cell gliosis: neuroprotective and detrimental effects. Prog Retin Eye Res 28(6):423–451
Taylor L, Arnér K, Ghosh F (2015) First responders: dynamics of pre-gliotic müller cell responses in the isolated adult rat retina. Curr Eye Res 40(12):1245–1260
Fontainhas AM, Townes-Anderson E (2011) RhoA inactivation prevents photoreceptor axon retraction in an in vitro model of acute retinal detachment. Invest Ophthalmol Vis Sci 52(1):579–587
Komaromy AM et al (2003) Long-term effect of retinal ganglion cell axotomy on the histomorphometry of other cells in the porcine retina. J Glaucoma 12:307–315
Mey J, Thanos S (1993) Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res 602:304–317
Watanabe M, Fukuda Y (2002) Survival and axonal regeneration of retinal ganglion cells in adult cats. Prog Retin Eye Res 21(6):529–553
Yao XY, Hageman GS, Marmor MF (1994) Retinal adhesiveness in the monkey. Invest Ophthalmol Vis Sci 35(2):744–748
Jackson TL et al (2003) An experimental model of rhegmatogenous retinal detachment: surgical results and glial cell response. Invest Ophthalmol Vis Sci 44:4026–4034
Acknowledgments
The authors would like to thank Elise Markström, Erica Cumléus and Oscar Manouchehrian for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Funding
The following funding agencies and trusts provided financial support for the research included in this paper: The Faculty of Medicine, University of Lund, The Swedish Research Council, The Carmen and Bertil Regnér Foundation, The King Gustaf V and Queen Victoria Freemason Foundation, The Foundation of Debilitating Eye Diseases in Malmöhus County. The sponsors had no role in the design or conduct of this research.
Animal experiments
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted and with the ARVO statement on the use of animals in ophthalmic and vision research. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Supported by: The Faculty of Medicine, University of Lund, The Swedish Research Council, The Carmen and Bertil Regnér Foundation, The King Gustaf V and Queen Victoria Freemason Foundation, The Foundation of Debilitating Eye Diseases in former Malmöhus County.
Rights and permissions
About this article
Cite this article
Ghosh, F., Arnér, K. & Taylor, L. In vitro biomechanical modulation—retinal detachment in a box. Graefes Arch Clin Exp Ophthalmol 254, 475–487 (2016). https://doi.org/10.1007/s00417-015-3236-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-3236-3