Abstract
Purpose
Choroideremia (CHM) is a X-chromosomal disorder leading to blindness by progressive degeneration of choroid, retinal pigment epithelium (RPE), and retinal neurons. A current clinical gene therapy trial (NCT01461213) showed promising safety and efficacy data in a carefully selected patient population. The present study was performed to shed light on pre-treatment characteristics of a larger cohort of CHM patients using a high resolution multi-modal approach.
Methods
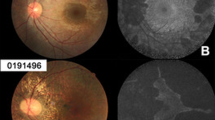
In a retrospective cross-sectional study, data from 58 eyes of 29 patients with clinically confirmed CHM were analysed including best-corrected visual acuity (BCVA), refractive error, spectral-domain optical coherence tomography (SD-OCT), fundus autofluorescence (FAF), perimetry, and tonometry. Residual retinal volume, area of residual RPE, and foveal thickness were quantified to further define natural disease progression and assess symmetry.
Results
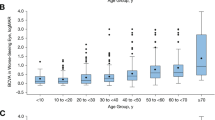
We evaluated 98 data points of BCVA [0.34 ± 0.06 (logMAR); mean ± 95 % confidence interval], 80 of IOP (14.6 ± 0.6 mmHg), and 98 of refraction (−2.16 ± 1.08 spherical equivalent). Visual fields (n = 76) demonstrated variable degrees of concentric constriction (54 % <10°, 25 % 10–30°, 21 % >30°). Mean residual RPE area on FAF (n = 64) measured 8.47 ± 1.91 mm2 (range 0.30–38.5 mm2), while mean neuroretinal volume (n = 42) was found to be 1.76 ± 0.12 mm3. Age at examination was exponentially associated with BCVA, while logarithmic functions best described progressive loss of retinal area and volume. A high degree of left to right symmetry was found in all modalities with structural markers showing the best correlation (r 2 area = 0.83; r 2 volume = 0.75).
Conclusion
Analysis of these widely available clinical data defines the natural disease characteristics of a relevant patient population eligible for gene therapeutic intervention. In the wake of preliminary reports on safety and efficacy of CHM gene therapy (NCT01461213), this multi-modal assessment of a cohort of CHM patients provides important evidence of the natural rate of disease progression and degree of symmetry between eyes.



Similar content being viewed by others
References
Mauthner L (1872) Ein Fall von Chorioideremie. Bericht des Naturwissenschaftlich-Medizinischen Vereins Innsbruck:191–197
Cremers FP, Brunsmann F, van de Pol TJ, Pawlowitzki IH, Paulsen K, Wieringa B, Ropers HH (1987) Deletion of the DXS165 locus in patients with classical choroideremia. Clin Genet 32:421–423
Cremers FP, van de Pol DJ, Diergaarde PJ, Wieringa B, Nussbaum RL, Schwartz M, Ropers HH (1989) Physical fine mapping of the choroideremia locus using Xq21 deletions associated with complex syndromes. Genomics 4:41–46
Cremers FP, van de Pol DJ, Wieringa B, Collins FS, Sankila EM, Siu VM, Flintoff WF, Brunsmann F, Blonden LA, Ropers HH (1989) Chromosomal jumping from the DXS165 locus allows molecular characterization of four microdeletions and a de novo chromosome X/13 translocation associated with choroideremia. Proc Natl Acad Sci U S A 86:7510–7514
Cremers FP, Sankila EM, Brunsmann F, Jay M, Jay B, Wright A, Pinckers AJ, Schwartz M, van de Pol DJ, Wieringa B et al (1990) Deletions in patients with classical choroideremia vary in size from 45 kb to several megabases. Am J Hum Genet 47:622–628
Cremers FP, Molloy CM, van de Pol DJ, van den Hurk JA, Bach I, Geurts van Kessel AH, Ropers HH (1992) An autosomal homologue of the choroideremia gene colocalizes with the Usher syndrome type II locus on the distal part of chromosome 1q. Hum Mol Genet 1:71–75
Cremers FP, Armstrong SA, Seabra MC, Brown MS, Goldstein JL (1994) REP-2, a Rab escort protein encoded by the choroideremia-like gene. J Biol Chem 269:2111–2117
Coussa RG, Traboulsi EI (2012) Choroideremia: a review of general findings and pathogenesis. Ophthalmic Genet 33:57–65
Rak A, Pylypenko O, Niculae A, Pyatkov K, Goody RS, Alexandrov K (2004) Structure of the Rab7:REP-1 complex: insights into the mechanism of Rab prenylation and choroideremia disease. Cell 117:749–760
Moosajee M, Ramsden SC, Black GC, Seabra MC, Webster AR (2014) Clinical utility gene card for: choroideremia. Eur J Hum Genet 22(4)
Wavre-Shapton ST, Tolmachova T, Lopes da Silva M, Futter CE, Seabra MC (2013) Conditional ablation of the choroideremia gene causes age-related changes in mouse retinal pigment epithelium. PLoS One 8:e57769
Tolmachova T, Tolmachov OE, Barnard AR, de Silva SR, Lipinski DM, Walker NJ, Maclaren RE, Seabra MC (2013) Functional expression of Rab escort protein 1 following AAV2-mediated gene delivery in the retina of choroideremia mice and human cells ex vivo. J Mol Med 91:825–837
MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, Clark KR, During MJ, Cremers FP, Black GC, Lotery AJ, Downes SM, Webster AR, Seabra MC (2014) Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet 383:1129–1137
Furgoch MJ, Mewes-Ares J, Radziwon A, Macdonald IM (2014) Molecular genetic diagnostic techniques in choroideremia. Mol Vis 20:535–544
Fischer MD, Fleischhauer JC, Gillies MC, Sutter FK, Helbig H, Barthelmes D (2008) A new method to monitor visual field defects caused by photoreceptor degeneration by quantitative optical coherence tomography. Invest Ophthalmol Vis Sci 49:3617–3621
Wiethoff S, Zhour A, Schols L, Fischer MD (2012) Retinal nerve fibre layer loss in hereditary spastic paraplegias is restricted to complex phenotypes. BMC Neurol 12:143
Fischer MD, Willmann G, Schatz A, Schommer K, Zhour A, Zrenner E, Bartz-Schmidt KU, Gekeler F (2012) Structural and functional changes of the human macula during acute exposure to high altitude. PLoS One 7:e36155
Bellmann C, Rubin GS, Kabanarou SA, Bird AC, Fitzke FW (2003) Fundus autofluorescence imaging compared with different confocal scanning laser ophthalmoscopes. Br J Ophthalmol 87:1381–1386
Holladay JT (1997) Proper method for calculating average visual acuity. J Refract Surg 13(4):388–391
Coussa RG, Kim J, Traboulsi EI (2012) Choroideremia: effect of age on visual acuity in patients and female carriers. Ophthalmic Genet 33:66–73
Roberts MF, Fishman GA, Roberts DK, Heckenlively JR, Weleber RG, Anderson RJ, Grover S (2002) Retrospective, longitudinal, and cross sectional study of visual acuity impairment in choroideraemia. Br J Ophthalmol 86:658–662
Bittner AK (2011) Variability in vision and photopsias in retinitis pigmentosa are related to disease severity and psychosocial factors. Dissertation. The Johns Hopkins University
Acknowledgments
The authors wish to acknowledge the help from all colleagues who helped obtain the clinical data on CHM patients in the “RP Sprechstunde” (outpatient clinic for hereditary retinal disorders) over previous decades. Drs. S Biskup, M Preisig, B Weber, and JA van den Hurk contributed to this work by verifying the diagnosis on a genetic level in some of the patients.
Conflict of interest statement
All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Grant support
Gesellschaft zur Förderung der Neuroophthalmologie e.V., Tistou & Charlotte Kerstan Foundation, Pro Retina e.V., UK Medical Research Council (MR/K003690/1);
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seitz, I.P., Zhour, A., Kohl, S. et al. Multimodal assessment of choroideremia patients defines pre-treatment characteristics. Graefes Arch Clin Exp Ophthalmol 253, 2143–2150 (2015). https://doi.org/10.1007/s00417-015-2976-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-2976-4