Abstract
Background
Microglial activation has been recognized as a neuropathological feature in diabetic retinopathy. But the early spatiotemporal characterization of microglial activation in the retina and the optic nerve of diabetic animals has not been fully investigated. The purpose of this study was to investigate early sequential changes of microglia in the retinas of rats with streptozotocin-induced diabetes. Microglia in the optic nerves of rats with streptozotocin-induced diabetes were also studied.
Methods
In 4-week, 8-week, and 12-week diabetic and normal control rats, microglial activation in the retinas and optic nerves was evaluated by immunolabeling with OX-42 antibody. Density, proportion of activation, and laminar distribution of retinal microglia were quantified. The retinal mRNA level of Iba-1, a microglial-specific marker, was measured by real-time PCR.
Results
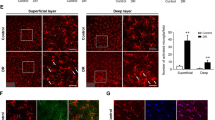
The density of retinal microglia was not different between diabetic and control rats, but the proportion of activated microglia increased significantly in diabetic rats at each time point. The proportion of microglia increased obviously in the nerve fiber layer and the ganglion cell layer while decreasing in the inner plexiform layer in 12-week diabetic rats. Moreover, retinal Iba-1 mRNA expression increased in 8-week and 12-week diabetic rats. Processes of microglia in the optic nerves of control rats were aligned with the long axis of nerve fibers, while the alignment was disturbed in diabetic rats.
Conclusions
Morphology, proportion of activation, distribution, and mRNA expression of retinal microglia changed characteristically with the progression of the disease in early-stage diabetic rats.






Similar content being viewed by others
References
Moss S, Klein R, Klein B (1998) The 14-year incidence of visual loss in a diabetic population. Ophthalmology 105:998–1003
Bunce C, Xing W, Wormald R (2010) Causes of blind and partial sight certifications in England and Wales: April 2007-March 2008. Eye (Lond) 24:1692–1699
Gardner T, Antonetti D, Barber A, LaNoue K, Levison S (2002) Diabetic retinopathy: more than meets the eye. Surv Ophthalmol 47(Suppl 2):S253–S262
Fletcher E, Phipps J, Wilkinson-Berka J (2005) Dysfunction of retinal neurons and glia during diabetes. Clin Exp Optom 88:132–145
Kern T (2007) Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res 2007:95103
Kim Y, Joh T (2006) Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp Mol Med 38:333–347
Zeng H, Green WR, Tso MOM (2008) Microglial activation in human diabetic retinopathy. Arch Ophthalmol 126:227–232
Rungger–Brändle E, Dosso AA, Leuenberger PM (2000) Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci 41:1971–1980
Gaucher D, Chiappore J-A, Pâques M, Simonutti M, Boitard C, Sahel J, Massin P, Picaud S (2007) Microglial changes occur without neural cell death in diabetic retinopathy. Vis Res 47:612–623
Zeng X, Y-k NG, E-a L (2000) Neuronal and microglial response in the retina of streptozotocin-induced diabetic rats. Vis Neurosci 11:463–471
Moss A, Beggs S, Vega-Avelaira D, Costigan M, Hathway G, Salter M, Fitzgerald M (2007) Spinal microglia and neuropathic pain in young rats. Pain 128:215–224
Sandhir R, Onyszchuk G, Berman N (2008) Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp Neurol 213:372–380
Robinson A, White T, Mason D (1986) Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRS OX-42, the latter recognising complement receptor type 3. Immunology 57:239–247
Davis E, Foster T, Thomas W (1994) Cellular forms and functions of brain microglia. Brain Res Bull 34:73–78
Raibon E, Sauvé Y, Carter D, Gaillard F (2002) Microglial changes accompanying the promotion of retinal ganglion cell axonal regeneration into peripheral nerve grafts. J Neurocytol 31:57–71
Hanisch U, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10:1387–1394
Streit W (2002) Microglia as neuroprotective, immunocompetent cells of the CNS. Glia 40:133–139
Krady J, Basu A, Allen C, Xu Y, LaNoue K, Gardner T, Levison S (2005) Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes 54:1559–1565
Arnal E, Miranda M, Johnsen-Soriano S, Alvarez-Nölting R, Díaz-Llopis M, Araiz J, Cervera E, Bosch-Morell F, Romero FJ (2009) Beneficial effect of docosahexanoic acid and lutein on retinal structural, metabolic, and functional abnormalities in diabetic rats. Curr Eye Res 34:928–938
Qiao H, Hisatomi T, Sonoda K, Kura S, Sassa Y, Kinoshita S, Nakamura T, Sakamoto T, Ishibashi T (2005) The characterisation of hyalocytes: the origin, phenotype, and turnover. Br J Ophthalmol 89:513–517
Marie O, Thillaye-Goldenberg B, Naud M, de Kozak Y (1999) Inhibition of endotoxin-induced Uveitis and potentiation of local TNF-a and interleukin-6 mRNA expression by interleukin-13. Invest Ophthalmol Vis Sci 40:2275–2282
Ohsawa K, Kohsaka S (2011) Dynamic motility of microglia: purinergic modulation of microglial movement in the normal and pathological brain. Glia 59:1793–1799
Ibrahim A, El-Remessy A, Matragoon S, Zhang W, Patel Y, Khan S, Al-Gayyar M, El-Shishtawy M, Liou G (2011) Retinal microglial activation and inflammation induced by amadori-glycated albumin in a rat model of diabetes. Diabetes 60:1122–1133
Hanisch U (2002) Microglia as a source and target of cytokines. Glia 40:140–155
Zeng H, Zhu X, Zhang C, Yang L, Wu L, Tso M (2005) Identification of sequential events and factors associated with microglial activation, migration, and cytotoxicity in retinal degeneration in rd mice. Invest Ophthalmol Vis Sci 46:2992–2999
Kowluru R, Odenbach S (2004) Role of interleukin-1beta in the development of retinopathy in rats: effect of antioxidants. Invest Ophthalmol Vis Sci 45:4161–4166
Demircan N, Safran B, Soylu M, Ozcan A, Sizmaz S (2006) Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye (Lond) 20:1366–1369
Kitaoka Y, Kitaoka Y, Kwong J, Ross-Cisneros F, Wang J, Tsai R, Sadun A, Lam T (2006) TNF-alpha-induced optic nerve degeneration and nuclear factor-kappaB p65. Invest Ophthalmol Vis Sci 47:1448–1457
Berger S, Savitz S, Nijhawan S, Singh M, David J, Rosenbaum P, Rosenbaum D (2008) Deleterious role of TNF-alpha in retinal ischemia-reperfusion injury. Invest Ophthalmol Vis Sci 49:3605–3610
Morgan S, Taylor D, Pocock J (2004) Microglia release activators of neuronal proliferation mediated by activation of mitogen-activated protein kinase, phosphatidylinositol-3-kinase/Akt and delta-Notch signalling cascades. J Neurochem 90:89–101
YZ L, CH L, FC C, CM H (2005) Molecular mechanisms responsible for microglia-derived protection of Sprague–Dawley rat brain cells during in vitro ischemia. Neurosci Lett 373:159–164
Yune T, Lee J, Jung G, Kim S, Jiang M, Kim Y, Oh Y, Markelonis G, Oh T (2007) Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. J Neurosci 27:7751–7761
Wang A, Yu A, Lau L, Lee C, Wu M, Zhu X, Tso M (2005) Minocycline inhibits LPS-induced retinal microglia activation. Neurochem Int 47:152–158
Chen M, Ona V, Li M, Ferrante R, Fink K, Zhu S, Bian J, Guo L, Farrell L, Hersch S, Hobbs W, Vonsattel J, Cha J, Friedlander R (2000) Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med 6:797–801
Wang J, Wei Q, Wang C, Hill W, Hess D, Dong Z (2004) Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem 279:19948–19954
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, X., Zhou, H., Gong, Y. et al. Early spatiotemporal characterization of microglial activation in the retinas of rats with streptozotocin-induced diabetes. Graefes Arch Clin Exp Ophthalmol 253, 519–525 (2015). https://doi.org/10.1007/s00417-014-2727-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2727-y