Abstract
Background
To characterize the effects of intravitreal injections of iodoacetic acid (IAA) in comparison to its systemic application as a measure to induce unilateral photoreceptor degeneration.
Methods
Seven-week-old C57BL/6 J mice received either intravitreal injections of IAA or systemic treatment (intraperitoneal vs intravenous) and were observed in the following 5 weeks using ERG, OCT, and histology.
Results
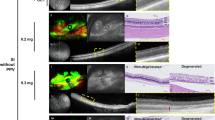
Systemic treatment with IAA induced high toxic effects and a high mortality in contrast to the intravitreal injection. Intraperitoneal application had no effect on the retina. Intravenous application of 2 × 30 mg/kg BW IAA (time between injections 3.5 h) resulted in an extinction of the ERG and a thinning of the retina, in particular of the outer nuclear layer (ONL) indicating photoreceptor degeneration. Animals receiving intravitreal injections developed cataracts already at low concentrations (up to 100 % at 0.25 mg/kg BW). Higher intravitreal IAA doses led to extinguished ERGs. In histology, a thinning of the entire retina was observed that was most prominent in the inner part of the retina.
Conclusions
In contrast to intraperitoneal administration, intravenous application of IAA led to a selective photoreceptor degeneration. After intravitreal injection, dense cataracts were already observed at concentrations lower than those needed to induce changes in the ERG. ERG results must be interpreted carefully. A thinning of all retinal layers rather than a specific outer retinal degeneration was observed upon intravitreal injection. IAA is not a useful model to induce outer retinal degeneration in mice.








Similar content being viewed by others
References
Delyfer MN, Forster V, Neveux N, Picaud S, Léveillard T, Sahel JA (2005) Evidence for glutamate-mediated excitotoxic mechanisms during photoreceptor degeneration in the rd1 mouse retina. Mol Vis 11:688–696
Hart AW, McKie L, Morgan JE, Gautier P, West K, Jackson IJ, Cross SH (2005) Genotype-phenotype correlation of mouse pde6b mutations. Invest Ophthalmol Vis Sci 46:3443–3450
Ahuja S, Ahuja-Jensen P, Johnson LE, Caffé AR, Abrahamson M, Ekström PA, van Veen T (2008) Rd1 Mouse retina shows an imbalance in the activity of cysteine protease cathepsins and their endogenous inhibitor cystatin C. Invest Ophthalmol Vis Sci 49:1089–1096
Gibson R, Fletcher EL, Vingrys AJ, Zhu Y, Vessey KA, Kalloniatis M (2013) Functional and neurochemical development in the normal and degenerating mouse retina. J Comp Neurol 521:1251–1267
Gargini C, Terzibasi E, Mazzoni F, Strettoi E (2007) Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. J Comp Neurol 500:222–238
Chen M, Wang K, Lin B (2012) Development and degeneration of cone bipolar cells are independent of cone photoreceptors in a mouse model of retinitis pigmentosa. PLoS One 7:e44036
Samardzija M, Wariwoda H, Imsand C, Huber P, Heynen SR, Gubler A, Grimm C (2012) Activation of survival pathways in the degenerating retina of rd10 mice. Exp Eye Res 99:17–26
Chrenek MA, Dalal N, Gardner C, Grossniklaus H, Jiang Y, Boatright JH, Nickerson JM (2012) Analysis of the RPE sheet in the rd10 retinal degeneration model. Adv Exp Med Biol 723:641–647
Cang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR (2002) Retinal degeneration mutants in the mouse. Vision Res 42:517–525
Chang B, Hawes NL, Pardue MT, German AM, Hurd RE, Davisson MT, Nusinowitz S, Rengarajan K, Boyd AP, Sidney SS, Phillips MJ, Stewart RE, Chaudhury R, Nickerson JM, Heckenlively JR, Boatright JH (2007) Two mouse retinal degenerations caused by missence mutations in the beta-subunit of rod cGMP phosphodiesterase gene. Vision Res 47:624–633
Pang JJ, Boye SL, Kumar A, Dinculescu A, Deng W, Li J, Li Q, Rani A, Foster TC, Chang B, Hawes NL, Boatright JH, Hauswirth WW (2008) AAV-mediated gene therapy for retinal degeneration in the rd10 mouse containing a recessive PDEbeta mutation. Invest Ophthalmol Vis Sci 49:4278–4283
Hess HH, Newsome DA, Knapka JJ, Westney GE (1982) Slitlamp assessment of age of onset and incidence of cataracts in pink-eyed, tan-hooded retinal dystrophic rats. Curr Eye Res 2:265–269
Matuk Y (1984) Retinitis pigmentosa and retinal degeneration in animals: a review. Can J Biochem Cell Biol 62:535–546
Fletcher EL, Kalloniatis M (1997) Neurochemical development of the degenerating rat retina. J Comp Neurol 388:1–22
Baid R, Upadhyay AK, Shinohara T, Kompella UB (2013) Biosynthesis, characterization, and efficacy in retinal degenerative diseases of lens epithelium-derived growth factor fragment (LEDGF1-326), a novel therapeutic protein. J Biol Chem 288:17372–17383
Nishida K, Kamei M, Kondo M, Sakaguchi H, Suzuki M, Fujikado T, Tano Y (2010) Efficacy of suprachoroidal-transretinal stimulation in a rabbit model of retinal degeneration. Invest Ophthalmol Vis Sci 51:2263–2268
Jones BW, Kondo M, Terasaki H, Watt CB, Rapp K, Anderson J, Lin Y, Shaw MV, Yang JH, Marc RE (2011) Retinal remodeling in the Tg P347L rabbit, a large-eye model of retinal degeneration. J Comp Neurol 519:2713–2733
Muraoka Y, Ikeda HO, Nakano N, Hangai M, Toda Y, Okamoto-Furuta K, Kohda H, Kondo M, Terasaki H, Kakizuka A, Yoshimura N (2012) Real-time imaging of rabbit retina with retinal degeneration by using spectral-domain optical coherence tomography. PLoS One 7:e36135
Hirota R, Kondo M, Ueno S, Sakai T, Koyasu T, Terasaki H (2012) Photoreceptor and post-photoreceptoral contributions to photopic ERG a-wave in rhodopsin P347L transgenic rabbits. Invest Ophthalmol Vis Sci 53:1467–1472
Harada T, Harada C, Sekiguchi M, Wada K (1998) Light-induced retinal degeneration suppresses developmental progression of flip-to-flop alternative splicing in GluR1. J Neurosci 18:3336–3343
Tsubura A, Yoshizawa K, Kuwata M, Uehara N (2010) Animal models for retinitis pigmentosa induced by MNU; disease progression, mechanisms and therapeutic trials. Histol Histopathol 25:933–944
Liang L, Katagiri Y, Franco LM, Yamauchi Y, Enzmann V, Kaplan HJ, Sandell JH (2008) Long-term cellular and regional specificity of the photoreceptor toxin, iodoacetic acid (IAA), in the rabbit retina. Vis Neurosci 25:167–177
Noell WK (1953) Experimentally induced toxic effects on structure and function of visual cells and pigment epithelium. Am J Ophthalmol 36:103–116
Sugimoto S, Imawaka M, Imai R, Kanamaru K, Ito T, Sasaki S, Ando T, Saijo T, Sato T (1997) A procedure for electroretinogram (ERG) recording in mice — effect of monoiodoacetic acid on the ERG in pigmented mice. J Toxicol Sci 2:315–325
Winkler BS, Sauer MW, Starnes CA (2003) Modulation of the Pasteur effect in retinal cells: implications for understanding compensatory metabolic mechanisms. Exp Eye Res 76:715–723
Matsumoto M, Morita T, Hirayama S, Uga S, Shimizu K (2002) Morphological analysis of Iodoacetic acid-induced cataract in the rat. Ophthalmic Res 34:119–127
Graymore C, Tansley K (1959) Iodoacetate poisoning of the rat retina. I. Production of retinal degeneration. Br J Ophthalmol 43:177–185
Graymore C, Tansley K (1959) Iodoacetate poisoning of the rat retina. II. Glycolysis in the poisoned retina. Br J Ophthalmol 43:486–493
Farber DB, Souza DW, Chase DG (1983) Cone visual cell degeneration in ground squirrel retina: disruption of morphology and cyclic nucleotide metabolism by Iodoacetic acid. Invest Ophthalmol Vis Sci 24:1236–1249
Cibis PA, Constant M, Pribyl A, Becker B (1957) Ocular lesions produced by Iodoacetate. AMA Arch Ophthalmol 57:508–519
Lasansky A, De Robertis E (1959) Submicroscopic changes in visual cells of the rabbit induced by iodoacetate. J Biophys Biochem Cytol 5:245–250
Orzalesi N, Calabria GA, Grignolo A (1970) Experimental degeneration of the rabbit retina induced by iodoacetic acid. a study of the ultrastructure, the rhodopsin cycle and the uptake of 14C-labeled iodoacetic acid. Exp Eye Res 9:246–253
Tieri O, Vecchione L (1963) Monolateral cataract provoked with iodoacetic acid. Acta Ophthalmol (Copenh) 41:205–212
Yamauchi Y, Agawa T, Tsukahara R, Kimura K, Yamakawa N, Miura M, Goto H (2011) Correlation between high-resolution optical coherence tomography (OCT) images and histopathology in an iodoacetic acid-induced model of retinal degeneration in rabbits. Br J Ophthalmol 95:1157–1160
Wang W, Fernandez de Castro J, Vukmanic E, Zhou L, Emery D, Demarco PJ, Kaplan HJ, Dean DC (2011) Selective rod degeneration and partial cone inactivation characterize an iodoacetic acid model of Swine retinal degeneration. Invest Ophthalmol Vis Sci 52:7917–7923
Scott PA, Kaplan HJ, Sandell JH (2011) Anatomical evidence of photoreceptor degeneration induced by iodoacetic acid in the porcine eye. Exp Eye Res 93:513–527
Noell WK (1952) The impairment of visual cell structure by iodoacetate. J Cell Physiol 40:25–55
Rösch S, Johnen S, Müller F, Pfarrer C, Walter P (2014) Correlations between ERG, OCT and anatomical findings in the rd10 mouse. J Ophthalmol [Epub Jan 19]. doi:10.1155/2014/874751
Marmor MF, Fulton AB, Holder GE, Miyake Y, Brigell M, Bach M (2009) ISCEV Standard for full-field clinical electroretinography (2008 update). Doc Ophthalmol 118:69–77
Mataruga A, Kremmer E, Müller F (2007) Type 3a and type 3b OFF cone bipolar cells provide for the alternative rod pathway in the mouse retina. J Comp Neurol 502:1123–1137
Eisenfeld AJ, Bund-Milam AH, Sarthy PV (1984) Müller cell expression of glial fibrillary acidic protein after genetic and experimental photoreceptor degeneration in the rat retina. Invest Ophthalmol Vis Sci 25(11):1321–1328
Nusinowitz S, Heckenlively JR (2006) Evaluating retinal function in the mouse retina with the electroretinogram. In: Heckenlively JR, Arden GB (eds) Principles and practice of clinical electrophysiology of vision, 2nd edn. MIT Press, London, England, pp 899–910
Acknowledgments
This work was supported by the DFG grants WA 1472/6–1 to PW and MU 3036/3–1 to FM. The authors thank Christoph Aretzweiler (Institute of Complex Systems, Cellular Biophysics, ICS-4, Forschungszentrum Jülich GmbH, 52428 Jülich, Germany) for the excellent support in preparation and staining of the eyes.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is part of a doctoral thesis at the University of Veterinary Medicine Hannover (December 2013).
Rights and permissions
About this article
Cite this article
Rösch, S., Johnen, S., Mazinani, B. et al. The effects of iodoacetic acid on the mouse retina. Graefes Arch Clin Exp Ophthalmol 253, 25–35 (2015). https://doi.org/10.1007/s00417-014-2652-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2652-0