Abstract
Purpose
To investigate factors influencing exudation recurrence following cataract surgery in patients already treated with anti-vascular endothelial growth factor (VEGF) agents for exudative age-related macular degeneration (AMD).
Methods
A retrospective review of medical records was performed for patients who underwent cataract surgery and had been previously treated with anti-VEGF for exudative AMD. Visual acuity was examined before surgery and 1 and 6 months after surgery. The time between diagnosis and surgery, and the exudation-free period before surgery were examined and compared between patients who had exudation recurrence and those that did not.
Results
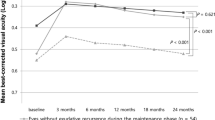
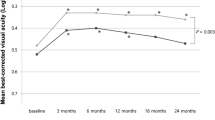
Thirty-nine eyes of 39 patients were included in analyses. The logarithm of the minimum angle of resolution visual acuity was 1.02 ± 0.58 and had significantly improved 1 month (0.81 ± 0.62, P < 0.001) and 6 months (0.85 ± 0.64, P = 0.001) following surgery. Both the diagnosis-to-surgery period (P = 0.001) and the preoperative exudation-free period (P < 0.001) were significantly longer in patients without recurrence than in patients with recurrence.
Conclusions
Cataract surgery was beneficial in patients previously treated with anti-VEGF for exudative AMD. Our data suggests that cataract surgery should be performed after a sufficiently long exudation-free period to minimize exudation recurrence. But larger prospective studies are required to draw definitive clinical guidelines.


Similar content being viewed by others
References
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355:1419–1431
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S (2006) Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 355:1432–1444
Spaide RF, Laud K, Fine HF, Klancnik JM Jr, Meyerle CB, Yannuzzi LA, Sorenson J, Slakter J, Fisher YL, Cooney MJ (2006) Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina 26:383–390
Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ, Puliafito CA, Davis JL, Flynn HW Jr, Esquiabro M (2007) An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol 143:566–583
Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ (2011) Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 364:1897–1908
Tabandeh H, Chaudhry NA, Boyer DS, Kon-Jara VA, Flynn HW Jr (2012) Outcomes of cataract surgery in patients with neovascular age-related macular degeneration in the era of anti-vascular endothelial growth factor therapy. J Cataract Refract Surg 38:677–682
Muzyka-Wozniak M (2011) Phacoemulsification in eyes with neovascular AMD treated with anti-VEGF injections. Eur J Ophthalmol 21:766–770
Rosenfeld PJ, Shapiro H, Ehrlich JS, Wong P (2011) Cataract surgery in ranibizumab-treated patients with neovascular age-related macular degeneration from the phase 3 ANCHOR and MARINA trials. Am J Ophthalmol 152:793–798
Arias L, Armada F, Donate J, Garcia-Arumi J, Giralt J, Pazos B, Pinero A, Martinez F, Mondejar JJ, Ortega I, Zlateva G, Buggage R (2009) Delay in treating age-related macular degeneration in Spain is associated with progressive vision loss. Eye (Lond) 23:326–333
Oliver-Fernandez A, Bakal J, Segal S, Shah GK, Dugar A, Sharma S (2005) Progression of visual loss and time between initial assessment and treatment of wet age-related macular degeneration. Can J Ophthalmol 40:313–319
Rauch R, Weingessel B, Maca SM, Vecsei-Marlovits PV (2012) Time to first treatment: the significance of early treatment of exudative age-related macular degeneration. Retina 32:1260–1264
Muether PS, Hoerster R, Hermann MM, Kirchhof B, Fauser S (2013) Long-term effects of ranibizumab treatment delay in neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 251:453–458
Saleh M, Kheliouen M, Tebeanu E, Ballonzoli L, Bourcier T, Speeg-Schatz C, Gaucher D (2013) Retreatment by series of three intravitreal injections of ranibizumab in neovascular age-related macular degeneration: long-term outcomes. Graefes Arch Clin Exp Ophthalmol 251:1901–1907
Muniraju R, Ramu J, Sivaprasad S (2013) Three-year visual outcome and injection frequency of intravitreal ranibizumab therapy for neovascular age-related macular degeneration. Ophthalmologica 230:27–33
Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W, Davis JL, Flynn HW Jr, Esquiabro M (2009) A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol 148:43–58
Ersoy L, Caramoy A, Ristau T, Kirchhof B, Fauser S (2013) Aqueous flare is increased in patients with clinically significant cystoid macular oedema after cataract surgery. Br J Ophthalmol 97:862–865
Alio JL, Sayans JA, Chipont E (1997) Flare-cell meter measurement of inflammation after uneventful cataract surgery with intraocular lens implantation. J Cataract Refract Surg 23:935–939
Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, Reeves BC (2012) Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 119:1399–1411
Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL 3rd (2012) Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 119:1388–1398
Conflict of interest
None
Meeting presentation
None
Financial support
This study is supported by Kim’s Eye Hospital Research Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, T.G., Kim, J.H., Chang, Y.S. et al. Factors influencing the exudation recurrence after cataract surgery in patients previously treated with anti-vascular endothelial growth factor for exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 252, 1573–1579 (2014). https://doi.org/10.1007/s00417-014-2624-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2624-4