Abstract
Objective
To determine the safety and efficacy of topical 0.03 % tacrolimus ointment treatment for subepithelial corneal infiltrates (SEIs).
Methods
This prospective non-controlled interventional case series included patients with SEIs who had been previously treated with topical corticosteroids with either no improvement or the medication being withdrawn due to associated intraocular pressure (IOP) elevation. The patients were treated with 0.03 % tacrolimus ointment twice daily for 22 weeks (including a 1-month washout). The objective data were best-corrected Snellen visual acuity (BCVA), IOP, and full ocular examination results, including SEI severity and the Schirmer test. The subjective data were the patients’ responses to a questionnaire at all follow-up visits.
Results
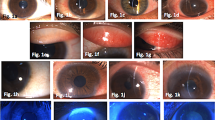
The patients consisted of five males (45 %) and six females (55 %) (mean age 50 ± 11 years) who were followed up for an average of 22 weeks. The mean BCVA (logarithm of the minimum angle of resolution [logMAR]) before and after treatment was 0.34 ± 0.09 and 0.08 ± 0.04 respectively (p = 0.042). All the patients evidenced significant objective clinical improvement, and none had a severe degree of SEI at the end of the treatment. The patients reported considerable reduction in the severity of their symptoms (foreign body sensation, glare, etc.). Three patients were excluded due to side-effects (one had severe dizziness and discomfort), and their data were excluded from the study.
Conclusion
Topical tacrolimus 0.03 % is a safe and effective alternative treatment in patients with SEIs who do not respond to other treatment modalities or have untoward side-effects from topical steroids.



Similar content being viewed by others
References
Hillenkamp J, Reinhard T, Ross RS, Böhringer D, Cartsburg O, Roggendorf M, De Clercq E, Godehardt E, Sundmacher R (2002) The effects of cidofovir 1% with and without cyclosporin A 1% as a topical treatment of acute adenoviral keratoconjunctivitis: a controlled clinical pilot study. Ophthalmology 109:845–850
Gordon YJ, Araullo-Cruz T, Romanowski EG (1998) The effects of topical nonsteroidal anti-inflammatory drugs on adenoviral replication. Arch Ophthalmol 116:900–905
Hillenkamp J, Reinhard T, Ross RS, Böhringer D, Cartsburg O, Roggendorf M, De Clercq E, Godehardt E, Sundmacher R (2001) Topical treatment of acute adenoviral keratoconjunctivitis with 0.2% cidofovir and 1% cyclosporine: a controlled clinical pilot study. Arch Ophthalmol 119:1487–1491
Lund OE, Stefani FH (1978) Corneal histology after epidemic keratoconjunctivitis. Arch Ophthalmol 96:2085–2088
Sahin A, Bozkurt B, Irkec M (2008) Topical cyclosporine a in the treatment of superior limbic keratoconjunctivitis: a long-term follow-up. Cornea 27:193–195
Ozcan AA, Ersoz TR, Dulger E (2007) Management of severe allergic conjunctivitis with topical cyclosporin a 0.05% eyedrops. Cornea 26:1035–1038
Doan S, Gabison E, Abitbol O, Gatinel D, Chast F, Hoang-Xuan T (2007) Efficacy of topical 2% cyclosporine A as a steroid-sparing agent in steroid-dependent vernal keratoconjunctivitis. J Fr Ophtalmol 30:697–701
Kino T, Hatanaka H, Hashimoto M, Nishiyama M, Goto T, Okuhara M, Kohsaka M, Aoki H, Imanaka H (1987) FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J Antibiot 40:1249–1255
Gallego-Pinazo R, Dolz-Marco R, Martínez-Castillo S, Arévalo JF, Díaz-Llopis M (2013) Update on the principles and novel local and systemic therapies for the treatment of non-infectious uveitis. Inflamm Allergy Drug Targets 12:38–45
Dhaliwal JS, Mason BF, Kaufman SC (2008) Long-term use of topical tacrolimus (FK506) in high-risk penetrating keratoplasty. Cornea 27:488–493
Meyer-Rüsenberg B, Loderstädt U, Richard G, Kaulfers P-M, Gesser C (2011) Epidemic keratoconjunctivitis: the current situation and recommendations for prevention and treatment. Dtsch Arztebl Int 108:475–480
Shiuey Y, Ambati BK, Adamis AP (2000) A randomized, double-masked trial of topical ketorolac versus artificial tears for treatment of viral conjunctivitis. Ophthalmology 107:1512–1517
Kowalski RP, Foulks GN, Gordon YJ (2000) Comparison of treatment regimens for ocular infections: community vs academic practice. Ann Ophthalmol 32:295–300
Romanowski EG, Yates KA, Gordon YJ (2002) Topical corticosteroids of limited potency promote adenovirus replication in the Ad5/NZW rabbit ocular model. Cornea 21:289–291
Romanowski EG, Pless P, Yates KA, Gordon YJ (2005) Topical cyclosporine A inhibits subepithelial immune infiltrates but also promotes viral shedding in experimental adenovirus models. Cornea 24:86–91
Lucchina S, Parvex SL, Biegger P, Fusetti C (2009) FK-506 ointment: an effective adjuvant therapy to treat a dramatic case of pyoderma gangrenosum of unilateral hand. Chin J Traumatol 12:181–183
Lauerma AI, Granlund H, Reitamo S (1997) Use of the newer immunosuppressive agents in dermatology. BioDrugs 8:96–106
Caffier PP, Harth W, Mayelzadeh B, Haupt H, Sedlmaier B (2007) Tacrolimus: a new option in therapy-resistant chronic external otitis. Laryngoscope 117:1046–1052
Kymionis GD, Kankariya VP, Kontadakis GA (2012) Tacrolimus ointment 0.03% for treatment of refractory childhood phlyctenular keratoconjunctivitis. Cornea 31:950–952
Attas-Fox L, Barkana Y, Iskhakov V, Rayvich S, Gerber Y, Morad Y, Avni I, Zadok D (2008) Topical tacrolimus 0.03% ointment for intractable allergic conjunctivitis: an open-label pilot study. Curr Eye Res 33:545–549
MedLine Plus (2013) Tacrolimus TM Drug Insert. National Institutes of Health (nlm.nih.gov)
Ebihara N, Ohashi Y, Fujishima H, Fukushima A, Nakagawa Y, Namba K, Okamoto S, Shoji J, Takamura E, Uchio E, Miyazaki D (2012) Blood level of tacrolimus in patients with severe allergic conjunctivitis treated by 0.1% tacrolimus ophthalmic suspension. Allergol Int 61:275–282
Ohashi Y, Ebihara N, Fujishima H, Fukushima A, Kumagai N, Nakagawa Y, Namba K, Okamoto S, Shoji J, Takamura E, Hayashi K (2010) A randomized, placebo-controlled clinical trial of tacrolimus ophthalmic suspension 0.1% in severe allergic conjunctivitis. J Ocul Pharmacol Ther 26:165–174
Conflict of interest
The authors did not receive any financial support from any public or private sources. The authors have no financial or proprietary interest in a product, method, or material described herein.
Author information
Authors and Affiliations
Corresponding author
Additional information
Drs. Levinger and Trivizki contributed equally to this work
Rights and permissions
About this article
Cite this article
Levinger, E., Trivizki, O., Shachar, Y. et al. Topical 0.03 % tacrolimus for subepithelial infiltrates secondary to adenoviral keratoconjunctivitis. Graefes Arch Clin Exp Ophthalmol 252, 811–816 (2014). https://doi.org/10.1007/s00417-014-2611-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2611-9