Abstract
Background
To assess the expression of toll-like receptors (TLRs) in human amniotic membrane (AM) specimens and compare this expression with those of AMs undergoing the standard preservation procedure (handling) for ocular surgery.
Methods
Human fresh (n = 10; five spontaneous and five cesarean) or handled (n = 5) AMs were analyzed for TLR gene and protein expression. Two pieces were obtained from each specimen, and subjected to molecular or biochemical analysis. Relative real-time PCR and SDS-PAGE were carried out according to standard procedures. The REST–ANOVA coupled analysis was used to compare the molecular and biochemical data.
Results
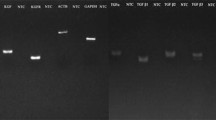
The fresh membranes expressed all the TLRs (TLR1-10), with different gene expression as detected/evidenced by the Ct values, the intra-fresh group analysis showing that there was a variation of TLR expression whichvaried within the fresh membranes. The handled AMs retained the TLR expression after standard processing and preservation, but with a particular pattern which included a high TLR3/TLR4 and low TLR6 expression, when compared to the fresh membranes. The molecular data were confirmed by Western blot analysis.
Conclusions
AM is routinely used in several ophthalmic surgical procedures, and notwithstanding its preservation procedure, AM is reported to favour wound healing and exert anti-angiogenic, anti-inflammatory, anti-scarring as well as anti-bacterial activities. The presence of TLRs in handled AM would imply that TLRs might be preserved in AMs used in ocular surgery. The findings herein described provide additional data concerning the presence of TLRs in cryopreserved AM, and suggest a possible contribution of AM in ocular surgery, via the innate immune response.


Similar content being viewed by others
References
Tseng SC, Prabhasawat P, Lee SH (1997) Amniotic membrane transplantation for conjunctival surface reconstruction. Am J Ophthalmol 124:765–774
Sippel KC, Ma J, Foster CS (2001) Amniotic membrane surgery. Curr Opin Ophthalmol 12:269–281
Bouchard CS, John T (2004) Amniotic membrane transplantation in the management of severe ocular surface disease: indications and outcomes. Ocul Surf 2:201–211
Dua HS, Gomes JA, King AJ, Maharajan VS (2004) The amniotic membrane in ophthalmology. Surv Ophthalmol 49:51–77
Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, Botelho MF (2012) Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res 349:447–458
Chen HJ, Pires RT, Tseng SC (2000) Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br J Ophthalmol 84:826–833
Hao Y, Ma DH-K, Hwang DG, Kim WS, Zhang F (2000) Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea 19:348–352
King WJ, Comer RM, Hudde T, Larkin DF, George AJ (2000) Cytokine and chemokine expression kinetics after corneal transplantation. Transplantation 70:1225–1233
Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S (2000) Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res 19:113–129
Koizumi N, Inatomi T, Sotozono C, Fullwood NJ, Quantock AJ, Kinoshita S (2000) Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res 20:173–177
Touhami A, Grueterich M, Tseng SC (2002) The role of NGF signaling in human limbal epithelium expanded by amniotic membrane culture. Invest Ophthalmol Vis Sci 43:987–994
Tseng SC, Espana EM, Kawakita T, Di Pascuale MA, Li W, He H, Liu TS, Cho TH, Gao YY, Yeh LK, Liu CY (2004) How does amniotic membrane work? Ocul Surf 2:177–187
Coassin M, Lambiase A, Micera A, Tirassa P, Aloe L, Bonini S (2006) Nerve growth factor modulates in vitro the expression and release of TGF-beta1 by amniotic membrane. Graefes Arch Clin Exp Ophthalmol 244:485–491
Hopkinson A, McIntosh RS, Tighe PJ, James DK, Dua HS (2006) Amniotic membrane for ocular surface reconstruction: donor variations and the effect of handling on TGF-beta content. Invest Ophthalmol Vis Sci 47:4316–4322
Ricci E, Vanosi G, Lindenmair A, Hennerbichler S, Peterbauer-Scherb A, Wolbank S, Cargnoni A, Signoroni PB, Campagnol M, Gabriel C, Redl H, Parolini O (2012) Anti-fibrotic effects of fresh and cryopreserved human amniotic membrane in a rat liver fibrosis model. Cell Tissue Bank. doi:10.1007/s10561-012-9337-x
Cho BJ, Djalilian AR, Obritsch WF, Matteson DM, Chan CC, Holland EJ (1999) Conjunctival epithelial cells cultured on human amniotic membrane fail to transdifferentiate into corneal epithelial-type cells. Cornea 18:216–224
Meller D, Tseng SC (1999) Conjunctival epithelial cell differentiation on amniotic membrane. Invest Ophthalmol Vis Sci 40:878–886
Kim JS, Kim JC, Na BK, Jeong JM, Song CY (2000) Amniotic membrane patching promotes healing and inhibits proteinase activity on wound healing following acute corneal alkali burn. Exp Eye Res 70:329–337
Park WC, Tseng SC (2000) Modulation of acute inflammation and keratocyte death by suturing blood and amniotic membrane in PRK. Invest Ophthalmol Vis Sci 41:2906–2914
Solomon A, Rosenblatt M, Monroy DC, Ji Z, Pflugfelder SC, Tseng SC (2001) Suppression of Interleukin-1α and Interleukin-1β in the human corneal epithelial cells cultured on the amniotic membrane matrix. Br J Ophthalmol 85:444–449
Madhira SL, Vemuganti G, Bhaduri A, Gaddipati S, Sangwan VS, Ghanekar Y (2008) Culture and characterization of oral mucosal epithelial cells on human amniotic membrane for ocular surface reconstruction. Mol Vis 14:189–196
Shimmura S, Shimazaki J, Ohashi Y, Tsubota K (2001) Antiinflammatory effects of amniotic membrane transplantation in ocular surface disorders. Cornea 20:408–413
Kawai T, Akira S (2010) Toll-like receptor signalling. Nat Immunol 11:373–384
Patni S, Flynn P, Wynen LP, Seager AL, Morgan G, White JO, Thornton CA (2007) An introduction to Toll-like receptors and their possible role in the initiation of labour. BJOG 114:1326–1334
Tseng SC, Meller D, Anderson DF, Touhami A, Pires RT, Grüterich M, Solomon A, Espana E, Sandoval H, Ti SE, Goto E (2002) Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane for treating corneal diseases with total limbal stem cell deficiency. Adv Exp Med Biol 506:1323–1334
Nagase H, Okugawa S, Ota Y, Yamaguchi M, Tomizawa H, Matsushima K, Ohta K, Yamamoto K, Hirai K (2003) Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. Immunol 171:3977–3982
Micera A, Lambiase A, Stampachiacchiere B, Sgrulletta R, Normando EM, Bonini S, Bonini S (2007) Nerve growth factor has a modulatory role on human primary fibroblast cultures derived from vernal keratoconjunctivitis-affected conjunctiva. Mol Vis 13:981–987
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:e36
Patni S, Wynen LP, Seager AL, Morgan G, White JO, Thornton CA (2009) Expression and activity of Toll-like receptors 1-9 in the human term placenta and changes associated with labor at term. Biol Reprod 80:243–248
Abrahams VM, Potter JA, Bhat G, Peltier MR, Saade G, Menon R (2013) Bacterial modulation of human fetal membrane toll-like receptor expression. Am J Reprod Immunol 69:33–40
Liu J, Sheha H, Fu Y, Liang L, Tseng SC (2010) Update on amniotic membrane transplantation. Expert Rev Ophthalmol 5:645–661
Lee SH, Tseng SC (1997) Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol 123:303–312
Azuara-Blanco A, Pillai CT, Dua HS (1999) Amniotic membrane transplantation for ocular surface reconstruction. Br J Ophthalmol 83:399–402
Nubile M, Dua HS, Lanzini TE, Carpineto P, Ciancaglini M, Toto L, Mastropasqua L (2008) Amniotic membrane transplantation for the management of corneal epithelial defects: an in vivo confocal microscopic study. Br J Ophthalmol 92:54–60
Fukada K, Chikama T, Nakamura M, Nishida T (1999) Differential distribution of subchains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea, and conjunctiva. Cornea 18:73–79
Hopkinson A, McIntosh RS, Shanmuganathan V, Tighe PJ, Dua HS (2006) Proteomic analysis of amniotic membrane prepared for human transplantation: characterization of proteins and clinical implications. J Proteome Res 5:2226–2235
Gillaux C, Méhats C, Vaiman D, Cabrol D, Breuiller-Fouché M (2011) Functional screening of TLRs in human amniotic epithelial cells. J Immunol 187:2766–2774
Kjaergaard N, Hein M, Hyttel L, Helmig RB, Schønheyder HC, Uldbjerg N, Madsen H (2001) Antibacterial properties of human amnion and chorion in vitro. Eur J Obstet Gynecol Reprod Biol 94:224–229
Gicquel JJ, Bejjani RA, Ellies P, Mercié M, Dighiero P (2007) Amniotic membrane transplantation in severe bacterial keratitis. Cornea 26:27–33
Lambiase A, Micera A, Sacchetti M, Mantelli F, Bonini S (2011) Toll-like receptors in ocular surface diseases: overview and new findings. Clin Sci (Lond) 120:441–450
Micera A, Stampachiacchiere B, Normando EM, Lambiase A, Bonini S, Bonini S (2009) Nerve growth factor modulates toll-like receptor (TLR) 4 and 9 expression in cultured primary VKC conjunctival epithelial cells. Mol Vis 15:2037–2044
Hopkinson A, Shanmuganathan VA, Gray T, Yeung AM, Lowe J, James DK, Dua HS (2008) Optimization of amniotic membrane (AM) denuding for tissue engineering. Tissue Eng Part C Methods 14:371–381
Imanishi T, Hara H, Suzuki S, Suzuki N, Akira S, Saito T (2007) Cutting edge: TLR2 directly triggers Th1 effector functions. J Immunol 178:6715–6719
Acknowledgments
The work was partially supported by the Italian “5×1000” tax contribution to IRCCS G.B. Bietti Foundation (2009; Rome). KJ was supported by PRVOUK-P24/LF1/3 program of the Charles University in Prague and by research project LM 2010004 BBMRI_CZ.
Financial Support
In part supported by the Italian “5×1000” tax contribution to IRCCS G.B. Bietti Foundation (Rome).
Conflict of Interest
None.
Presentation at conferences
None.
Clinical Trial registration number
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Micera, A., Jirsova, K., Normando, E.M. et al. Molecular and biochemical expression of TLRs in human amniotic membrane: a comparative study of fresh and cryopreserved specimens. Graefes Arch Clin Exp Ophthalmol 252, 267–274 (2014). https://doi.org/10.1007/s00417-013-2540-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-013-2540-z