Abstract
Purpose
To analyse the long-term functional and morphological response of a specific choroidal neovascular membrane (CNV) phenotype to anti-vascular endothelial growth factor (VEGF) therapy.
Methods
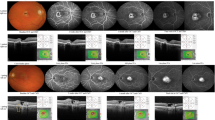
Data from 30 eyes of 30 consecutive patients with subretinal fluid (SRF) and fibrovascular pigment epithelial detachment (PED) due to CNV on spectral-domain optical coherence tomography (SDOCT) with a follow-up of at least 20 months were retrospectively collected. Main outcome measures included change in visual acuity, quantitative and qualitative parameters on SDOCT [photoreceptor layer, outer nuclear layer (ONL), choroid, PED, SRF] and on fluorescein angiography (CNV activity). Subjects were divided into responders and non-responders based on morphological and functional aspects.
Results
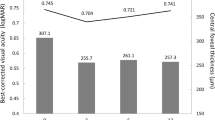
An average number of 20.23 ± 9.9 anti-VEGF injections were administered during a mean follow-up of 40.25 ± 13.5 months. Fourteen eyes were categorized as morphological non-responders, 12 as functional non-responders and eight as complete non-responders. Complete non-responders were significantly younger than complete responders (68.5 ± 4.5 vs 74.3 ± 6.8 years; p < 0.05) and presented thinner baseline ONL values (68.43 ± 15.2 vs103.5 ± 32.8 μm; p < 0.05). Intermediate or large drusen as typical features for age-related macular degeneration (AMD) were less frequently present in complete non-responders; however, this was not statistically significant (62.5 % vs 91.7 %; p = 0.25).
Conclusions
Our preliminary findings indicate that eyes with the specific SDOCT phenotype with isolated fibrovascular PED and SRF frequently demonstrate non-response to anti-VEGF therapy, and the underlying disease mechanism may be different from AMD. Larger prospective trials are required to validate those results, and to develop strategies to improve the morphological as well as functional outcome.

Similar content being viewed by others
References
Bressler NM, Bressler SB, Congdon NG, Ferris FL 3rd, Friedman DS, Klein R, Lindblad AS, Milton RC, Seddon JM, Age-Related Eye Disease Study Research G (2003) Potential public health impact of age-related eye disease study results: AREDS report no. 11. Arch Ophthalmol 121:1621–1624
Shah AR, Del Priore LV (2009) Natural history of predominantly classic, minimally classic, and occult subgroups in exudative age-related macular degeneration. Ophthalmology 116:1901–1907
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S, Group AS (2006) Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 355:1432–1444
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, Group MS (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355:1419–1431
Comparison of Age-related Macular Degeneration Treatments Trials Research G, Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL 3rd (2012) Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: Two-year results. Ophthalmology 119:1388–1398
Bressler NM, Doan QV, Varma R, Lee PP, Suner IJ, Dolan C, Danese MD, Yu E, Tran I, Colman S (2011) Estimated cases of legal blindness and visual impairment avoided using ranibizumab for choroidal neovascularization: Non-hispanic white population in the United States with age-related macular degeneration. Arch Ophthalmol 129:709–717
Konstantinidis L, Mantel I, Pournaras JA, Zografos L, Ambresin A (2009) Intravitreal ranibizumab (Lucentis) for the treatment of myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol 247:311–318
Lai TY, Chan WM, Liu DT, Lam DS (2009) Intravitreal ranibizumab for the primary treatment of choroidal neovascularization secondary to pathologic myopia. Retina 29:750–756
Mones JM, Amselem L, Serrano A, Garcia M, Hijano M (2009) Intravitreal ranibizumab for choroidal neovascularization secondary to pathologic myopia: 12-month results. Eye (Lond) 23:1275–1280, quiz 1281
Nguyen QD, Shah SM, Hafiz G, Do DV, Haller JA, Pili R, Zimmer-Galler IE, Janjua K, Symons RC, Campochiaro PA (2008) Intravenous bevacizumab causes regression of choroidal neovascularization secondary to diseases other than age-related macular degeneration. Am J Ophthalmol 145:257–266
Boyer DS, Antoszyk AN, Awh CC, Bhisitkul RB, Shapiro H, Acharya NR, Group MS (2007) Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology 114:246–252
Rosenfeld PJ, Shapiro H, Tuomi L, Webster M, Elledge J, Blodi B, MARINA and ANCHOR Groups (2011) Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology 118:523–530
Barbazetto I, Burdan A, Bressler NM, Bressler SB, Haynes L, Kapetanios AD, Lukas J, Olsen K, Potter M, Reaves A, Rosenfeld P, Schachat AP, Strong HA, Wenkstern A, Treatment of Age-Related Macular Degeneration with Photodynamic Therapy Study G, Verteporfin in Photodynamic Therapy Study G (2003) Photodynamic therapy of subfoveal choroidal neovascularization with verteporfin: Fluorescein angiographic guidelines for evaluation and treatment—TAP and VIP report No. 2. Arch Ophthalmol 121:1253–1268
Kaiser PK, Brown DM, Zhang K, Hudson HL, Holz FG, Shapiro H, Schneider S, Acharya NR (2007) Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol 144:850–857
Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ, Puliafito CA, Davis JL, Flynn HW Jr, Esquiabro M (2007) An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol 143:566–583
Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W, Davis JL, Flynn HW Jr, Esquiabro M (2009) A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: Year 2 of the PrONTO study. Am J Ophthalmol 148(43–58):e41
Keane PA, Patel PJ, Liakopoulos S, Heussen FM, Sadda SR, Tufail A (2012) Evaluation of age-related macular degeneration with optical coherence tomography. Surv Ophthalmol 57:389–414
Joeres S, Tsong JW, Updike PG, Collins AT, Dustin L, Walsh AC, Romano PW, Sadda SR (2007) Reproducibility of quantitative optical coherence tomography subanalysis in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 48:4300–4307
Witkin AJ, Vuong LN, Srinivasan VJ, Gorczynska I, Reichel E, Baumal CR, Rogers AH, Schuman JS, Fujimoto JG, Duker JS (2009) High-speed ultrahigh resolution optical coherence tomography before and after ranibizumab for age-related macular degeneration. Ophthalmology 116:956–963
Golbaz I, Ahlers C, Stock G, Schutze C, Schriefl S, Schlanitz F, Simader C, Prunte C, Schmidt-Erfurth UM (2011) Quantification of the therapeutic response of intraretinal, subretinal, and subpigment epithelial compartments in exudative AMD during anti-VEGF therapy. Invest Ophthalmol Vis Sci 52:1599–1605
Keane PA, Liakopoulos S, Ongchin SC, Heussen FM, Msutta S, Chang KT, Walsh AC, Sadda SR (2008) Quantitative subanalysis of optical coherence tomography after treatment with ranibizumab for neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 49:3115–3120
Liakopoulos S, Ongchin S, Bansal A, Msutta S, Walsh AC, Updike PG, Sadda SR (2008) Quantitative optical coherence tomography findings in various subtypes of neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 49:5048–5054
Ristau T, Hillebrand S, Smailhodzic D, Walsh AC, Kirchhof B (2013) Prognostic factors for long term visual acuity outcome after ranibizumab therapy in patients with neovascular age-related macular degeneration. J Clin Exp Ophthalmol 4:264
Singh RP, Fu EX, Smith SD, Williams DR, Kaiser PK (2009) Predictive factors of visual and anatomical outcome after intravitreal bevacizumab treatment of neovascular age-related macular degeneration: an optical coherence tomography study. Br J Ophthalmol 93:1353–1358
Ritter M, Elledge J, Simader C, Deak GG, Benesch T, Blodi BA, Schmidt-Erfurth UM (2011) Evaluation of optical coherence tomography findings in age-related macular degeneration: A reproducibility study of two independent reading centres. Br J Ophthalmol 95:381–385
Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ (2006) Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology 113:363.e365–372.e365
Spaide RF, Laud K, Fine HF, Klancnik JM Jr, Meyerle CB, Yannuzzi LA, Sorenson J, Slakter J, Fisher YL, Cooney MJ (2006) Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina 26:383–390
Heier JS, Antoszyk AN, Pavan PR, Leff SR, Rosenfeld PJ, Ciulla TA, Dreyer RF, Gentile RC, Sy JP, Hantsbarger G, Shams N (2006) Ranibizumab for treatment of neovascular age-related macular degeneration: a phase I/II multicenter, controlled, multidose study. Ophthalmology 113(633):e631–e634
Freeman WR, Kozak I, Yuson RM, Nigam N, Cheng L, Mojana F (2011) Prognosti implications of pigment epithelial detachment in bevacizumab (avastin)-treated eyes with age-related macular degeneration and choroidal neovascularization. Retina 31:1812–1818
Veritti D, Macor S, Menchini F, Lanzetta P (2013) Effects of VEGF inhibition on retinal morphology, neovascular network size, and visual acuity in patients with vascularized pigment epithelium detachment because of occult choroidal neovascularization. Retina 33:982–989
Zweifel SA, Engelbert M, Laud K, Margolis R, Spaide RF, Freund KB (2009) Outer retinal tubulation: A novel optical coherence tomography finding. Arch Ophthalmol 127:1596–1602
Pappuru RR, Ouyang Y, Nittala MG, Hemmati HD, Keane PA, Walsh AC, Sadda SR (2011) Relationship between outer retinal thickness substructures and visual acuity in eyes with dry age-related macular degeneration. Invest Ophthalmol Vis Sci 52:6743–6748
Hee MR, Puliafito CA, Wong C, Reichel E, Duker JS, Schuman JS, Swanson EA, Fujimoto JG (1995) Optical coherence tomography of central serous chorioretinopathy. Am J Ophthalmol 120:65–74
Spaide RF, Noble K, Morgan A, Freund KB (2006) Vitelliform macular dystrophy. Ophthalmology 113:1392–1400
Brecher R, Bird AC (1990) Adult vitelliform macular dystrophy. Eye (Lond) 4(Pt 1):210–215
Gass JD (1967) Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol 63(Suppl):1–139
Loo RH, Scott IU, Flynn HW Jr, Gass JD, Murray TG, Lewis ML, Rosenfeld PJ, Smiddy WE (2002) Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina 22:19–24
Imamura Y, Fujiwara T, Margolis R, Spaide RF (2009) Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina 29:1469–1473
Kim YT, Kang SW, Bai KH (2011) Choroidal thickness in both eyes of patients with unilaterally active central serous chorioretinopathy. Eye (Lond) 25:1635–1640
Kuroda S, Ikuno Y, Yasuno Y, Nakai K, Usui S, Sawa M, Tsujikawa M, Gomi F, Nishida K (2013) Choroidal thickness in central serous chorioretinopathy. Retina 33:302–308
Chung SE, Kang SW, Lee JH, Kim YT (2011) Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology 118:840–845
Kloos P, Niederberger H, Valmaggia C (2011) Photodynamic therapy in “secondary sick RPE syndrome” after repeated intravitreal injections of VEGF inhibitors in patients with wet age-related macular degeneration. Klin Monatsbl Augenheilkd 228:340–344
Joeres S, Kaplowitz K, Brubaker JW, Updike PG, Collins AT, Walsh AC, Romano PW, Sadda SR (2008) Quantitative comparison of optical coherence tomography after pegaptanib or bevacizumab in neovascular age-related macular degeneration. Ophthalmology 115:347.e342–354.e342
Eghoj MS, Sorensen TL (2012) Tachyphylaxis during treatment of exudative age-related macular degeneration with ranibizumab. Br J Ophthalmol 96:21–23
Forooghian F, Cukras C, Meyerle CB, Chew EY, Wong WT (2009) Tachyphylaxis after intravitreal bevacizumab for exudative age-related macular degeneration. Retina 29:723–731
Gasperini JL, Fawzi AA, Khondkaryan A, Lam L, Chong LP, Eliott D, Walsh AC, Hwang J, Sadda SR (2012) Bevacizumab and ranibizumab tachyphylaxis in the treatment of choroidal neovascularisation. Br J Ophthalmol 96:14–20
Patel KH, Chow CC, Rathod R, Mieler WF, Lim JI, Ulanski LJ 2nd, Leiderman YI, Arun V, Chau FY (2013) Rapid response of retinal pigment epithelial detachments to intravitreal aflibercept in neovascular age-related macular degeneration refractory to bevacizumab and ranibizumab. Eye (Lond) 27:663–667, quiz 668
Yonekawa Y, Andreoli C, Miller JB, Loewenstein JI, Sobrin L, Eliott D, Vavvas DG, Miller JW, Kim IK (2013) Conversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degeneration. Am J Ophthalmol 156(29–35):e22
Bakall B, Folk JC, Boldt HC, Sohn EH, Stone EM, Russell SR, Mahajan VB (2013) Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab. Am J Ophthalmol 156:15.e11–22.e11
Kumar N, Marsiglia M, Mrejen S, Fung AT, Slakter J, Sorenson J, Freund KB (2013) Visual and anatomical outcomes of intravitreal aflibercept in eyes with persistent subfoveal fluid despite previous treatments with ranibizumab in patients with neovascular age-related macular degeneration. Retina 33(8):1605–1612
Hirami Y, Mandai M, Takahashi M, Teramukai S, Tada H, Yoshimura N (2009) Association of clinical characteristics with disease subtypes, initial visual acuity, and visual prognosis in neovascular age-related macular degeneration. Jpn J Ophthalmol 53:396–407
Acknowledgments
Supported in part by Ilse Palm-Foundation, Germany
Conflict of Interest
Lebriz Ersoy: none, Tina Ristau: none, Sandra Liakopoulos served as a consultant for Novartis Pharma and received honoraria from Novartis Pharma, Bernd Kirchhof: none
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ersoy, L., Ristau, T., Kirchhof, B. et al. Response to anti-VEGF therapy in patients with subretinal fluid and pigment epithelial detachment on spectral-domain optical coherence tomography. Graefes Arch Clin Exp Ophthalmol 252, 889–897 (2014). https://doi.org/10.1007/s00417-013-2519-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-013-2519-9