Abstract
Background
To keep the loss of endothelial cell density in donor corneas to a minimum, a storage medium which is adjusted to their nutritional needs is necessary. Different media, used either serum-supplemented or serum-free, are available. The quality of medium- and serum-batches as well as support of endothelial cell viability by the medium are to be tested with a quality assured screening system that allows routine examination.
Methods
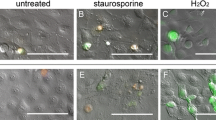
A screening system was developed which is based on cell-culture tests with the well-established human corneal endothelial cell line HCEC-12, and therefore can be performed without the need for donor corneas. The cells are plated at a defined density in cell-culture dishes, and are cultured for a defined period of time in the test media. Evaluation is carried out by assaying cell count, activity of cell metabolism (resazurin conversion), and determining the number of apoptotic and necrotic cells (combined vital staining with YO-PRO®-1/propidium iodide and subsequent flow cytometry).
Results
Human corneal endothelial cells that are cultured in a medium which is adjusted to their nutritional needs achieve higher cell numbers and show a higher metabolic rate. Simultaneously, the percentage of apoptotic and necrotic cells is lower. The screening system developed in this study allows for easy and reliable detection of slightest differences between different media, different processing steps for same media, and different supplements, as well as different serum batches.
Conclusions
The differentiated results show that the screening system is sensitive enough to show even minor quality differences. Therefore, it is more suitable than the hitherto commonly used growth assay with primary, mostly porcine, corneal endothelial cells.



Similar content being viewed by others
References
Waring GO, Bourne WM, Edelhauser HF, Kenyon KR (1982) The corneal endothelium. Normal and pathologic structure and function. Ophthalmology 89:531–590
Bahn CF, Glassman RM, MacCallum DK, Lillie JH, Meyer RF, Robinson BJ, Rich NM (1986) Postnatal development of corneal endothelium. Invest Ophthalmol Vis Sci 27:44–51
Bourne WM, Kaufman HE (1976) The endothelium of clear corneal transplants. Arch Ophthalmol 94:1730–1732
Thuret G, Chiquet C, Bernal F, Acquart S, Romanet JP, Mouillon M, Hegelhoffer H, Burillon C, Damour O, Maugery J, Armitage WJ, Gain P (2003) Prospective, randomized clinical and endothelial evaluation of 2 storage times for cornea donor tissue in organ culture at 31 °C. Arch Ophthalmol 121(4):442–450
Armitage WJ, Easty DL (1997) Factors influencing the suitability of organ-cultured corneas for transplantation. Invest Ophthalmol Vis Sci 38:16–24
Pels E, Schuchard Y (1983) Organ-culture preservation of human corneas. Doc Ophthalmol 56:147–153
Møller-Pedersen T, Hartmann U, Møller HJ, Ehlers N, Engelmann K (2001) Evaluation of potential organ culture media for eye banking using human donor corneas. Br J Ophthalmol 85:1075–1079
Gavrilov J, Borderie VM, Laroche L, Delbosc B (2010) Influencing factors on the suitability of organ-cultured corneas. Eye (Lond) 24:1227–1233
Jackel T, Knels L, Valtink M, Funk RHW, Engelmann K (2011) Serum-free corneal organ culture medium (SFM) but not conventional minimal essential organ culture medium (MEM) protects human corneal endothelial cells from apoptotic and necrotic cell death. British J Ophthalmol 95(1):123–130
Bednarz J (2001) Effect of three different media on serum free culture of donor corneas and isolated human corneal endothelial cells. British J Ophthalmol 85:1416–1420
Hempel B, Bednarz J, Engelmann K (2001) Use of a serum-free medium for long-term storage of human corneas. Influence on endothelial cell density and corneal metabolism. Graefes Arch Clin Exp Ophthalmol 239:801–805
Moller-Pedersen T, Hartmann U, Ehlers N, Engelmann K (2001) Evaluation of potential organ culture media for eye banking using a human corneal endothelial cell growth assay. Graefes Arch Clin Exp Ophthalmol 239:778–782
Moller-Pedersen T, Hartmann U, Moller HJ, Ehlers N, Engelmann K (2001) Evaluation of potential organ culture media for eye banking using human donor corneas. Br J Ophthalmol 85:1075–1079
Engelmann K, Sobottka Ventura A, Drexler D, Staude HJ (1998) A sensitive method for testing the quality of organ culture media and of individual medium components in a cornea bank. Graefes Arch Clin Exp Ophthalmol 236:312–319
Thuret G (2005) Animal compound-free medium and poloxamer for human corneal organ culture and deswelling. Invest Ophthalmol Vis Sci 46:816–822
Engelmann K, Winter R (1993) Quality control in the corneal bank—a necessary measure? Klin Monatsbl Augenheilkd 203:262–268
Zamai L, Falcieri E, Marhefka G, Vitale M (1996) Supravital exposure to propidium iodide identifies apoptotic cells in the absence of nucleosomal DNA fragmentation. Cytometry 23(4):303–311
Zamai L, Canonico B, Luchetti F, Ferri P, Melloni E, Guidotti L, Cappellini A, Cutroneo G, Vitale M, Papa S (2001) Supravital exposure to propidium iodide identifies apoptosis on adherent cells. Cytometry 44:57–64
The European Parliament and the Council of the European Union (2004) Directive 2004/23/EC of the European parliament and of the council of 31 March 2004 on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage, and distribution of human tissues and cells. Off J Eur Comm L102:48–58
The European Parliament and the Council of the European Union (2006) Commission Directive 2006/17/EC of 8 February 2006 implementing directive 2004/23/EC of the European parliament and of the council as regards certain technical requirements for the donation, procurement and testing of human tissues and cells. Off J Eur Comm L38:40–52
Acknowledgments
This work was partially supported by DGFG (German Society for Tissue Transplantation, Hannover, Germany).
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Presentation at a conference: 110. DOG-Kongress, September 20–23 2012, Berlin
Rights and permissions
About this article
Cite this article
Schönfelder, J., Valtink, M., Knels, L. et al. Quality assessment of corneal storage media and their components. Graefes Arch Clin Exp Ophthalmol 252, 77–82 (2014). https://doi.org/10.1007/s00417-013-2482-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-013-2482-5