Abstract
Background
The extent of retinal tissue deformation by histological processing needs to be separately measured for every workup protocol. This work presents a simple approach for its quantitative assessment, and shows lateral and axial scaling factors for a common protocol. We calibrated histological measurements by in-vivo photographic and optical coherence tomographic (OCT) measurements, using retinal photocoagulation lesions as calibration markers.
Methods
We evaluated four rabbit eyes that were examined histologically after fixation in Margo’s solution (1 % paraformaldehyde:1.25 % glutaraldehyde), isopropanol dehydration, paraffin embedding and hematoxylin and eosin staining. Distances between 51 pairs of laser lesions were compared in photographs and on histological slides. Retinal thickness measurements were performed at 15 anatomically defined sites in these eyes, and related to anatomically matched OCT thickness measurements of six different rabbit eyes.
Results
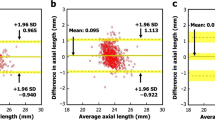
We found that the ratio of histological over photographic lesion distances was 1.17 (95 % CI 1.13–1.22), indicating 17 % lateral retinal stretching or expansion by the processing. Thickness measurements in histology were 65.6 % of the in-vivo thickness as measured in OCT, indicating 1/3 axial tissue compression or shrinkage.
Conclusions
We provide an analysis of retinal tissue deformation after fixation in Margo’s solution and paraffin embedding. In spite of protocol optimization for reduced tissue deformation, the workup caused 1/3 axial compression/shrinkage and 17 % lateral elongation, which was unexpected. We show a simple way how to calibrate retina specimens by fundus photography and OCT, two methods that are readily available to most ophthalmologists. Our findings underline the necessity to calibrate specimens prior to morphometry.






Similar content being viewed by others
References
Oberholzer M (1983) Morphometrie in der klinischen Pathologie. Allgemeine Grundlagen. Springer, Berlin Heidelberg New York
Quintyn JC, Brasseur G (2004) Subretinal fluid in primary rhegmatogenous retinal detachment: physiopathology and composition. Surv Ophthalmol 49:96–108
Margo CE, Lee A (1995) Fixation of whole eyes: the role of fixative osmolarity in the production of tissue artifact. Graefes Arch Clin Exp Ophthalmol 233:366–370
Koinzer S, Schlott K, Ptaszynski L, Bever M, Kleemann S, Saeger M, Baade A, Caliebe A, Miura Y, Birngruber R, Brinkmann R, Roider J (2012) Temperature-controlled retinal photocoagulation — a step toward automated laser treatment. Invest Ophthalmol Vis Sci 53:3605–3614
Schlott K, Koinzer S, Ptaszynski L, Bever M, Baade A, Roider J, Birngruber R, Brinkmann R (2012) Automatic temperature-controlled retinal photocoagulation. J Biomed Opt 17:061223
Prince JH (1964) The rabbit in eye research. Charles Thomas, Springfield
Stefánsson E, Wilson CA, Lightman SL, Kuwabara T, Palestine AG, Wagner HG (1987) Quantitative measurements of retinal edema by specific gravity determinations. Invest Ophthalmol Vis Sci 28:1281–1289
Watson PG, Young RD (2004) Scleral structure, organisation and disease. A review. Exp Eye Res 78:609–623
Yu AK, Merrill KD, Truong SN, Forward KM, Morse LS, Telander DG (2013) The comparative histologic effects of subthreshold 532 nm and 810 nm diode micropulse laser on the retina. Invest Ophthalmol Vis Sci 54(3):2216–2224. doi:10.1167/iovs.12-11382
Hoerster R, Muether PS, Vierkotten S, Schröder S, Kirchhof B, Fauser S (2012) In-vivo and ex-vivo characterization of laser-induced choroidal neovascularization variability in mice. Graefes Arch Clin Exp Ophthalmol 250:1579–1586
Bahr GF, Bloom G, Friberg U (1957) Volume changes of tissues in physiological fluids during fixation in osmium tetroxide or formaldehyde and during subsequent treatment. Exp Cell Res 12:342–355
Davies DJ, Brewer DB, Hardwicke J (1978) Urinary proteins and glomerular morphometry in protein overload proteinuria. Lab Invest 38:232–243
Weibel ER (1979) Morphometry of the human lung: the state of the art after two decades. Bull Eur Physiopathol Respir 15:999–1013
Hatton WJ, von Bartheld CS (1999) Analysis of cell death in the trochlear nucleus of the chick embryo: calibration of the optical disector counting method reveals systematic bias. J Comp Neurol 409:169–186
Gardella D, Hatton WJ, Rind HB, Rosen GD, von Bartheld CS (2003) Differential tissue shrinkage and compression in the z-axis: implications for optical disector counting in vibratome-, plastic- and cryosections. J Neurosci Meth 124:45–59
Li Q, Onozato ML, Andrews PM, Chen CW, Paek A, Naphas R, Yuan S, Jiang J, Cable A, Chen Y (2009) Automated quantification of microstructural dimensions of the human kidney using optical coherence tomography (OCT). Opt Express 17:16000–16016
Mujat M, Greco K, Galbally-Kinney KL, Hammer DX, Ferguson RD, Iftimia N, Mulhall P, Sharma P, Pikal MJ, Kessler WJ (2012) Optical coherence tomography-based freeze-drying microscopy. Biomed Opt Express 3:55–63
Gambichler T, Jaedicke V, Terras S (2011) Optical coherence tomography in dermatology: technical and clinical aspects. Arch Dermatol Res 303:457–473
Regar E, Ligthart J, Bruining N, van Soest G (2011) The diagnostic value of intracoronary optical coherence tomography. Herz 36:417–429
Knott EJ, Sheets KG, Zhou Y, Gordon WC, Bazan NG (2011) Spatial correlation of mouse photoreceptor–RPE thickness between SD-OCT and histology. Exp Eye Res 92:155–160
Anger EM, Unterhuber A, Hermann B, Sattmann H, Schubert C, Morgan JE, Cowey A, Ahnelt PK, Drexler W (2004) Ultrahigh resolution optical coherence tomography of the monkey fovea. Identification of retinal sublayers by correlation with semithin histology sections. Exp Eye Res 78:1117–1125
Curcio CA, Messinger JD, Sloan KR, Mitra A, McGwin G, Spaide RF (2011) Human chorioretinal layer thicknesses measured in macula-wide, high-resolution histologic sections. Invest Ophthalmol Vis Sci 52:3943–3954
Abbott CJ, McBrien NA, Grünert U, Pianta MJ (2009) Relationship of the optical coherence tomography signal to underlying retinal histology in the tree shrew (Tupaia belangeri). Invest Ophthalmol Vis Sci 50:414–423
Baur R (1969) Error correction by sterologic measuring on human placenta. 2. Cutting compression. Experientia 25:1173
Paulus YM, Jain A, Gariano RF, Stanzel BV, Marmor M, Blumenkranz MS, Palanker D (2008) Healing of retinal photocoagulation lesions. Invest Ophthalmol Vis Sci 49:5540–5545
Birngruber R (1991) Choroidal circulation and heat convection at the fundus of the eye. In: Wolbarsht ML (ed) Laser applications in medicine and biology. Plenum Press, New York, pp 277–358
Verolino M, Nastri G, Sellitti L, Costagliola C (1999) Axial length increase in lid-sutured rabbits. Surv Ophthalmol 44(Suppl 1):103–108
Park HS, Kim JY, Shin JP, Choi YJ, Kim SY (2005) Effect of experimental scleral shortening on axial length of the rabbit eye. Korean J Ophthalmol 19:101–205
Acknowledgements
The authors declare that they have no conflict of interest.
The data were presented at the following conference:
• Annual meeting of the German-speaking Ophthalmopathologists, Erlangen, Germany; October 26/27, 2012.
The authors gratefully acknowledge grant support for this collaborative research project by the German Ministry of Education and Research (BMBF) according to the Innovation Award for Advancing Medical Technology 2006, grant #01EZ0734 (Dept. of Ophthalmology, University hospital of Schleswig-Holstein, Campus Kiel), #01EZ0732 (Medical Laser Center Lübeck), #01EZ0733 (Institute of Biomedical Optics Lübeck) and #01EZ0735 (Carl Zeiss Meditec AG).
Technical assistance by Monika Marquardt, Serap Luick, and Barbara Fluke is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was conducted in a collaborative project which was supported by the German Federal Ministry of Education and Research (BMBF).The authors have full control of all primary data, and they agree to allow Graefe's Archive for Clinical and Experimental Ophthalmology to review their data upon request.
Rights and permissions
About this article
Cite this article
Koinzer, S., Bajorat, S., Hesse, C. et al. Calibration of histological retina specimens after fixation in Margo’s solution and paraffin embedding to in-vivo dimensions, using photography and optical coherence tomography. Graefes Arch Clin Exp Ophthalmol 252, 145–153 (2014). https://doi.org/10.1007/s00417-013-2457-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-013-2457-6