Abstract
Background
Reactive oxygen species (ROS) play an important role in the pathogenesis of various ocular diseases. ROS can induce vasodilation or vasoconstriction depending on the species, the tested vessel bed, and the condition of the vessel. This study investigates the effect of different dosages of ROS on the tone of rat ophthalmic arteries.
Methods
Freshly dissected rat ophthalmic arteries were pressurized in a perfusion setup in steps of 10 mmHg to 180 mmHg in three consecutive cycles. The first cycle was run under mostly physiological conditions, the second cycle was run after ROS treatment, and the third cycle as passive dilation after all Ca2+ was removed from the solution. ROS-induced dilation or constriction was calculated in relation to the passive dilation. All experiments were performed with or without endothelium.
Results
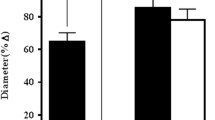
For vessels with endothelium, dilation in control experiments was 20.0 ± 0.1 %; after 5 s of ROS dilation was 74.4 ± 0.6 %, and after 20 s 87.4 ± 0.3 %. ANOVA revealed significant differences between these groups (P = 0.048). For vessels without endothelium, a slight dilation was seen in control experiments (14.5 ± 0.4 %), which was also present after 5 s of ROS treatment (15.4 ± 0.4 %). Treatment with ROS for 20 s led to a constriction of the vessel preparations (−16.6 ± 0.5 %; P = 0.831).
Conclusions
ROS led to a vasodilation in vessels with endothelium that was not seen in vessels without endothelium. Endothelial function seems to determine the effect of ROS on the vessel tone in isolated rat ophthalmic arteries.



Similar content being viewed by others
References
Alizai AM, Trobe JD, Thompson BG, Izer JD, Cornblath WT, Deveikis JP (2005) Ocular ischemic syndrome after occlusion of both external carotid arteries. J Neuroophthalmol 25:268–272
Cai J, Boulton M (2002) The pathogenesis of diabetic retinopathy: old concepts and new questions. Eye 16:242–260
Flammer J, Pache M, Resink T (2001) Vasospasm, its role in the pathogenesis of diseases with particular reference to the eye. Prog Retin Eye Res 20:319–349
Erdurmus M, Yagci R, Atis O, Karadag R, Akbas A, Hepsen IF (2011) Antioxidant status and oxidative stress in primary open angle glaucoma and pseudoexfoliative glaucoma. Curr Eye Res 36:713–718
Tezel G, Yang X, Luo C, Kain AD, Powell DW, Kuehn MH, Kaplan HJ (2010) Oxidative stress and the regulation of complement activation in human glaucoma. Invest Ophthalmol Vis Sci 51:5071–5082
Madsen-Bouterse SA, Kowluru RA (2008) Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Rev Endocr Metab Disord 9:315–327
Fernandes R, Hosoya K, Pereira P (2011) Reactive oxygen species downregulate glucose transport system in retinal endothelial cells. Am J Physiol 300:C927–C936
Auch-Schwelk W, Katusic ZS, Vanhoutte PM (1989) Contractions to oxygen-derived free radicals are augmented in aorta of the spontaneously hypertensive rat. Hypertension 13:859–864
Stewart DJ, Pohl U, Bassenge E (1988) Free radicals inhibit endothelium-dependent dilation in the coronary resistance bed. Am J Physiol 255:H765–H769
Rubanyi GM (1988) Vascular effects of oxygen-derived free radicals. Free Radic Biol Med 4:107–120
Zeitz O, Wagenfeld L, Wirtz N, Galambos P, Matthiesen N, Wiermann A, Richard G, Klemm M (2007) Influence of oxygen free radicals on the tone of ciliary arteries: a model of vasospasms of ocular vasculature. Graefes Arch Clin Exp Ophthalmol 245:1327–1333
Bharadwaj L, Prasad K (1995) Mediation of H2O2-induced vascular relaxation by endothelium-derived relaxing factor. Mol Cell Biochem 149–150:267–270
Wolin MS, Rodenburg JM, Messina EJ, Kaley G (1987) Oxygen metabolites and vasodilator mechanisms in rat cremasteric arterioles. Am J Physiol 252:H1159–H1163
Wagenfeld L, Himpel O, Galambos P, Matthiesen N, Wiermann A, Richard G, Klemm M, Zeitz O (2008) Protective effects of nebivolol on oxygen free radical-induced vasoconstrictions in vitro. Med Sci Monit 14:BR109–BR112
Wei EP, Kontos HA (1990) H2O2 and endothelium-dependent cerebral arteriolar dilation. Implications for the identity of endothelium-derived relaxing factor generated by acetylcholine. Hypertension 16:162–169
Sobey CG, Heistad DD, Faraci FM (1997) Mechanisms of bradykinin-induced cerebral vasodilatation in rats. Evidence that reactive oxygen species activate K+ channels. Stroke 28:2290–2294, discussion 2295
Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A (2000) Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest 106:1521–1530
Barlow RS, White RE (1998) Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am J Physiol 275:H1283–H1289
Thengchaisri N, Kuo L (2003) Hydrogen peroxide induces endothelium-dependent and -independent coronary arteriolar dilation: role of cyclooxygenase and potassium channels. Am J Physiol Heart Circ Physiol 285:H2255–H2263
Matoba T, Shimokawa H, Kubota H, Morikawa K, Fujiki T, Kunihiro I, Mukai Y, Hirakawa Y, Takeshita A (2002) Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem Biophys Res Commun 290:909–913
Rosenblum WI (1983) Effects of free radical generation on mouse pial arterioles: probable role of hydroxyl radicals. Am J Physiol 245:H139–H142
Rosenblum WI (1987) Hydroxyl radical mediates the endothelium-dependent relaxation produced by bradykinin in mouse cerebral arterioles. Circ Res 61:601–603
Todoki K, Okabe E, Ito H (1982) Free radical damage to mesenteric microcirculation in cats (Abstract). Microvasc Res 24:223
Wei EP, Kontos HA, Beckman JS (1996) Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. Am J Physiol 271:H1262–H1266
Lucchesi PA, Belmadani S, Matrougui K (2005) Hydrogen peroxide acts as both vasodilator and vasoconstrictor in the control of perfused mouse mesenteric resistance arteries. J Hypertens 23:571–579
Kellogg EW 3rd, Fridovich I (1975) Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J Biol Chem 250:8812–8817
Saran M, Bors W (1989) Oxygen radicals acting as chemical messengers: a hypothesis. Free Radic Res Commun 7:213–220
Yada T, Shimokawa H, Hiramatsu O, Kajita T, Shigeto F, Goto M, Ogasawara Y, Kajiya F (2003) Hydrogen peroxide, an endogenous endothelium-derived hyperpolarizing factor, plays an important role in coronary autoregulation in vivo. Circulation 107:1040–1045
Acknowledgment
This work was supported by a grant of the Ernst und Berta Grimmke Stiftung, the Claere Jung Stiftung, and the Werner Otto Stiftung to Lars Wagenfeld and Oliver Zeitz.
Conflict of interest
The authors declare that they have no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wagenfeld, L., von Domarus, F., Weiss, S. et al. The effect of reactive oxygen species on the myogenic tone of rat ophthalmic arteries with and without endothelium. Graefes Arch Clin Exp Ophthalmol 251, 2339–2344 (2013). https://doi.org/10.1007/s00417-013-2387-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-013-2387-3