Abstract
Background
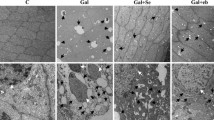
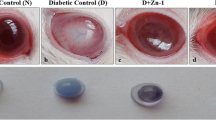
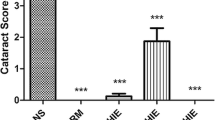
Cataract, the leading cause of blindness, is associated with oxidative damage and protein modification in the lens. The present study was carried out to assess the efficacy of rutin on rat-lens crystallins in selenite-induced in-vivo cataract models.
Methods
Eight-day-old Sprague–Dawley rat pups were grouped as control (G I), experimental (G II) and rutin-treated (G III). The rat pups in G II, and G III received a single subcutaneous injection of sodium selenite (4 μg/g body weight) and G I received a single subcutaneous injection of sterile water on the 10th day. The treatment groups (G III) were administered with rutin (1 μg/g body weight) respectively from the 8th to 15th day. Cataract was visualized from the 16th day. Lens crystallins (α, β, and γ) were isolated by size exclusion chromatography. Chaperone activity of isolated crystallins was measured by heat, DTT, and oxidation-induced aggregation and refolding assays. Concentration of total protein (soluble and insoluble) and SDS–PAGE analysis of soluble proteins were also done.
Results
Treatment with rutin prevented the loss of α crystallin chaperone property, and protein insolubilization prevailed during selenite-induced cataract.
Conclusions
These results suggest the therapeutic potential of rutin, a bioflavonoid, against selenite-induced cataract, which has been reported in this paper for the first time. The work assumes significance, as this is a novel approach in modulating the chaperone activity of lens crystallins in selenite-induced cataract by a natural product.








Similar content being viewed by others
References
Blomendal H (1982) Lens proteins. CRC Crit Rev Biochem 12:1–38
Wistow G, Piatigorsky J (1988) Lens crystallins: the evolution and the expression of proteins for highly specialized tissue. Ann Rev Biochem 57:479–504
Horwitz J (2003) Alpha-crystallin can function as a molecular chaperone. Exp Eye Res 76:145–148
Varma SD, Chand D, Sharma YR, Kuck JF Jr, Richards RD (1984) Oxidative stress on lens and cataract formation: role of light and oxygen. Curr Eye Res 3:35–37
Ohia SE, Opere CA, LeDay AM (2005) Pharmacological consequences of oxidative stress in ocular tissues. Mutat Res 579:22–36
Thampi P, Hassan A, Smith JB, Abraham EC (2002) Enhanced C-terminal truncation of alpha A and alpha B crystallins in diabetic lenses. Invest Ophthalmol Vis Sci 43(344):3265–3272
Hanson SR, Hasen A, Smith DL, Smith JB (2000) The major in vivo modification of the human water insoluble lens crystallins are disulfide bonds, deamidation, methionine oxidation and backbone cleavage. Exp Eye Res 71:195–207
Harding J (1991) Cataract: biochemistry, epidemiology and pharmacology, 1st edn. Chapman and Hall, New York
Ueda Y, Duncan MK, David LL (2002) Lens proteomics: the accumulation of crystallin modifications in the mouse lens with age. Invest Ophthalmol Vis Sci 43:205–215
Bockelbrink A, Roll S, Ruether K, Rasch A, Greiner W, Willich SN (2008) Cataract surgery and the development or progression of age-related macular degeneration: a systematic review. Surv Ophthalmol 53:359–367
Toh T, Morton J, Coxon J, Elder MJ (2007) Medical treatment of cataract. Clin Exp Ophthalmol 35:664–671
Toda J, Kato S, Oshika T, Sugita G (2007) Posterior capsule opacification after combined cataract surgery and vitrectomy. J Cataract Refract Surg 33:104–107
Cornish KM, Williamson G, Sanderson J (2002) Quercetin metabolism in the lens: role in inhibition of hydrogen peroxide induced cataract. Free Radic Biol Med 33:63–70
Gupta SK, Trivedi D, Srivastava S, Joshi S, Halder N, Verma SD (2003) Lycopene attenuates oxidative stress induced experimental cataract development: an in vitro and in vivo study. Nutrition 19:794–799
Gupta SK, Srivastava S, Trivedi D, Joshi S, Halder N (2005) Ocimum sanctum modulates selenite-induced cataractogenic changes and prevents rat lens opacification. Curr Eye Res 30:583–591
Lija Y, Biju PG, Reeni A, Cibin TR, Sahasranamam V, Abraham A (2006) Modulation of selenite cataract by the flavonoid fraction of Emilia sonchifolia in experimental animal models. Phytother Res 20:1091–1095
Elanchezhian R, Ramesh E, Sakthivel M, Isai M, Geraldine P (2007) Acetyl L-carnitine prevents selenite-induced cataractogenesis in an experimental animal model. Curr Eye Res 32:961–971
Biju PG, Devi VG, Lija Y, Abraham A (2007) Protection against selenite cataract in rat lens by drevogenin D, a triterpenoid aglycone from Dregea volubilis. J Med Food 10(2):308–315
Rooban BN, Lija Y, Biju PG, Sasikala V, Sahasranamam V, Abraham A (2009) Vitex nigundo attenuates calpain activation and cataractogenesis in selenite models. Exp Eye Res 88:575–582
Sasikala V, Rooban BN, Siva Priya SG, Sahasranamam V, Abraham A (2010) Moringa oleifera prevents selenite-induced cataractogenesis in rat pups. J Ocul Pharmacol Ther 26(5):441–447
Anwar F, Latif S, Ashraf M, Gilani AH (2007) Moringa oleifera: a food plant with multiple medicinal uses. Phytother Res 21:17–25
Sreelatha S, Padma PR (2009) Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum Nutr 64(4):303–311
Al-Rejaie SS, Abuohashish HM, Alkhamees OA, Aleisa AM, Alroujayee AS (2012) Gender difference following high cholesterol diet induced renal injury and the protective role of rutin and ascorbic acid combination in wistar rats. Lipids Health Dis 11:41
Isai M, Sakthivel M, Ramesh E, Thomas PA, Geraldine P (2009) Prevention of selenite-induced cataractogenesis by rutin in wistar rats. Mol Vis 15:2570–2577
Kyung TW, Lee JE, Shin HH, Choi HS (2008) Rutin inhibits osteoclast formation by decreasing reactive oxygen species and TNF-α by inhibiting activation of NFĸB. Exp Mol Med 40(1):52–58
Ostadalova I, Babicky A, Obenberger J (1978) Cataract induced by administration of a single dose of sodium selenite to suckling rats. Experientia 34:222–225
Hiraoka T, Clark JI (1995) Inhibition of lens opacification during the early stages of cataract formation. Invest Ophthalmol Vis Sci 36:2550–2555
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of Bacteriophage T4. Nature 227:680–685
Reddy GB, Das KP, Petrash JM, Surewicz WK (2000) Temperature dependent chaperone activity and structural properties of human alpha A and alpha B crystallins. J Biol Chem 275:4565–4570
Wang K, Spector A (1994) The chaperone activity of bovine alpha crystallin. Interaction with other lens crystallins in native and denatured states. J Biol Chem 269:13601–13608
Raman B, Rao CM (1997) Chaperone-like activity and temperature-induced structural changes of α-crystallin. J Biol Chem 272(38):23559–23564
Steel RGD, Torrie JH, Dickey DA (1997) Principles and procedures of statistics: a biometrical approach, 3rd edn. McGraw-Hill, New York
Babicky A, Rychter Z, Kopoldova J, Ostadalova I (1985) Age dependence of selenite uptake in rat eye lenses. Exp Eye Res 40:101–103
Shearer TR, Ma H, Fukiage C, Azuma M (1997) Selenite nuclear cataract: review of the model. Mol Vis 38:1–14
Matsushima H, David LL, Hiraoka T, Clark JI (1997) Loss of cytoskeletal proteins and lens cell opacification in the selenite cataract model. Exp Eye Res 64:387–395
Shearer TR, Shih M, Mizuno T, David L (1996) Crystallins from rat lens are especially susceptible to calpain-induced light scattering compared to other species. Curr Eye Res 15:860–868
Nakamura Y, Fukiage C, Shih M, Ma H, David LL, Azuma M, Shearer TR (2000) Contribution of calpain Lp82-induced proteolysis to experimental cataractogenesis in mice. Invest Ophthalmol Vis Sci 41:1460–1466
Yan H, Harding JJ, Hui YN, Li MY (2003) Decreased chaperone activity of α crystallin in selenite cataract may result from selenite-induced aggregation. Eye 17:637–645
Reddy GB, Reddy PY, Vijayalakshmi A, Kumar MS, Suryanarayana P, Sesikeran B (2002) Effect of long term dietary manipulation on the aggregation of rat lens crystallins: role of α-crystallin chaperone function. Mol Vis 8:298–305
Peluso G, Petillo O, Barbarisi A, Melone MAB, Reda E, Nicolai R, Calvani M (2001) Carnitine protects the molecular chaperone activity of lens α-crystallin and decreases the posttranslational protein modifications induced by oxidative stress. FASEB J 15(9):1604–1606. doi:10.1096/fj.00-0727fje
Srinivas V, Raman B, Rao KS, Ramakrishna T, Rao CM (2005) Arginine hydrochloride enhances the dynamics of subunit assembly and the chaperone-like activity of α-crystallin. Mol Vis 11:249–255
Hori Y, Yoshikawa T, Tsuji N, Bamba T, Aso Y, Kudou M, Uchida Y, Takagi M, Harada K, Hirata K (2009) Phytochelatins inhibit the metal-induced aggregation of α-crystallin. J Biosci Bioeng 107(2):173–186
Anilkumar P, Suryanarayana P, Reddy PY, Reddy GB (2005) Modulation of α-crystallin chaperone activity in diabetic rat lens by curcumin. Mol Vis 11:561–568
Wang K, Spector A (1995) α-crystallin can act as a chaperone under conditions of oxidative stress. Invest Opthalmol Vis Sci 36(2):311–321
Raman B, Ramakrishna T, Rao CM (1995) Temperature-dependent chaperone-like activity of alpha-crystallin. FEBS Lett 365:133–136
Boyle SP, Dobson VL, Duthie SJ, Hinselwood DC, Kyle JA, Collins AR (2000) Bioavailability and efficiency of rutin as an antioxidant: a human supplementation study. Eur J Clin Nutr 54:774–782
Acknowledgments
Financial assistance in the form of junior research fellowship to Ms. Sasikala V from CSIR, New Delhi, India is greatefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sasikala, V., Rooban, B.N., Sahasranamam, V. et al. Rutin ameliorates free radical mediated cataract by enhancing the chaperone activity of α-crystallin. Graefes Arch Clin Exp Ophthalmol 251, 1747–1755 (2013). https://doi.org/10.1007/s00417-013-2281-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-013-2281-z