Abstract
Purpose
To investigate the effects of cetuximab and bevacizumab on experimental rat model of corneal angiogenesis.
Methods
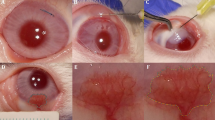
The right eyes of 28 male Sprague–Dawley rats were included in silver nitrate cauterization-induced corneal angiogenesis model. They were divided into four groups: (1) silver nitrate cauterization-induced and 0.15 ml serum physiologic was given to the angiogenesis group, (2) bevacizumab was given 1.25 mg to the bevacizumab group, (3) cetuximab was given 5 mg to the cetuximab group, and (4) 1.25 mg bevacizumab plus 5 mg cetuximab were given to the bevacizumab + cetuximab group. All eyes were exposed to the treatment on days 1, 4, and 7 of the experiment, and drugs were given subconjunctivally. The left eyes were untreated and used as sham. On day 8, the treated eyes were evaluated biomicroscopically. Then, the rats were sacrificed, and corneal specimens were prepared for histopathologic examinations.
Results
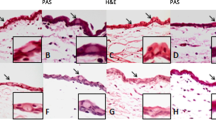
The degree of angiogenesis inhibition was observed as 50.8 % in bevacizumab, 54.3 % in cetuximab, and 15.8 % in bevacizumab + cetuximab groups by biomicroscopic evaluation. According to the histopathological and immunohistochemical findings obtained from the present study, the amount of angiogenesis was determined to have decreased considerably in both the bevacizumab and cetuximab groups; also, relatively less inhibiton was observed in the bevacizumab + cetuximab group.
Conclusion
Subconjunctival injection of cetuximab and bevacizumab is effective in reducing corneal angiogenesis in silver nitrate cauterization induced angiogenesis model of rats. Further investigation is needed to assess the potential side-effects of the drugs, especially cetuximab.




Similar content being viewed by others
References
Cursiefen C, Masli S, Ng TF, Dana MR, Bornstein P, Lawler J, Streilein JW (2004) Roles of thrombospondin-1 and -2 in regulating corneal and iris angiogenesis. Invest Ophthalmol Vis Sci 45:1117–1124
Cursiefen C, Maruyama K, Jackson DG, Streilein JW, Kruse FE (2006) Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea 25:443–447
Bakri SJ, Cameron JD, McCannel CA, Pulido JS, Marler RJ (2006) Absence of histologic retinal toxicity of intravitreal bevacizumab in a rabbit model. Am J Ophthalmol 142:162–164
Yamazaki Y, Takani K, Atoda H, Morita T (2003) Snake venom vascular endothelial growth factors (VEGFs) exhibit potent activity through their specific recognition of KDR (VEGF receptor-2). J Biol Chem 278:51985–51988
Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J (2000) Vascular-specific growth factors and blood vessel formation. Nature 407:242–248
Bock F, König Y, Dietrich T, Zimmermann P, Baier M, Cursiefen C (2007) Inhibition of angiogenesis in the anterior chamber of the eye. Ophthalmologe 104:336–344
Ferrara N, Davis Smyth T (1997) The biology of vascular endothelial growth factor. Endocr Rev 18:4–25
Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS (2005) Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology 112:1035–1047
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–2550
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350(23):2335–2342
Blick SK, Scott LJ (2007) Cetuximab: a review of its use in squamous cell carcinoma of the head and neck and metastatic colorectal cancer. Drugs 67(17):2585–2607
Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM (2005) Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 7(4):301–311
Green CJ (1982) Animal anaesthesia. Laboratory Animals Ltd., London, pp 45–58
Mahoney J, Waterbury LD (1985) Drug effects on the neovascularization response to silver nitrate cauterization of the rat cornea. Curr Eye Res 4(5):531–535
Carmeliet P (2005) Angiogenesis in life, disease and medicine. Nature 438:932–936
Carmeliet P (2003) Angiogenesis in health and disease. Nat Med 9:653–660
Ferrara N, Kerbel RS (2005) Angiogenesis as a therapeutic target. Nature 438:967–974
Shojaei F, Ferrara N (2007) Antiangiogenesis to treat cancer and intraocular neovascular disorders. Lab Invest 87:227–320
Lyn SS, Cheng CM (2007) Bevacizumab for neovascular ocular diseases. Ann Pharmacother 41:614–625
Joussen AM, Poulaki V, Mitsiades N, Stechschulte SU, Kirchhof B, Dartt DA, Fong GH, Rudge J, Wiegand SJ, Yancopoulos GD, Adamis AP (2003) VEGF-dependent conjunctivalization of the corneal surface. Invest Ophthalmol Vis Sci 44:117–123
Schrag D (2004) The price tag on progress–chemotherapy for colorectal cancer. N Engl J Med 351(4):317–319
Erdurmus M, Totan Y (2007) Subconjunctival bevacizumab for corneal neovascularization. Graefes Arch Clin Exp Ophthalmol 245(10):1577–9
Avery RL, Pieramici DJ, Rabena MD (2006) Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology 113:363–372
Spaide RF, Fisher YL (2006) Intravitreal bevacizumab(Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina 26:275–278
Manzano RP, Peyman GA, Khan P, Carvounis P, Kivilcim M (2007) Inhibition of experimental corneal neovascularisation by bevacizumab (Avastin). BrJ Ophthalmol 91:804–807
Dratviman-Storobinsky O, Lubin BC, Hasanreisoglu M, Goldenberg-Cohen N (2009) Effect of subconjuctival and intraocular bevacizumab injection on angiogenic gene expression levels in a mouse model of corneal neovascularization. Mol Vis 15:2326–2338
Bohling T, Hatva E, Kujala M, Claesson-Welsh L, Alitalo K, Haltia M (1996) Expression of growth factors and growth factor receptors in capillary hemangioblastoma. J Neuropathol Exp Neurol 55:522–7
Goldman CK, Kim J, Wong WL, King V, Brock T, Gillespie GY (1993) Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Mol Biol Cell 4:121–33
Bruns CJ, Solorzano CC, Harbison MT, Ozawa S, Tsan R, Fan D, Abbruzzese J, Traxler P, Buchdunger E, Radinsky R, Fidler IJ (2000) Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res 60:2926–2935
Mendelsohn J (2000) Blockade of receptors for growth factors: an anticancer therapy the fourth annual Joseph H Burchenal American Association of Cancer Research Clinical Research Award lecture. Clin Cancer Res 6:747–753
Kerbel RS, Viloria-Petit A, Okada F, Rak J (1998) Establishing a link between oncogenes and tumor angiogenesis. Mol Med 4:286–295
Guler M, Yilmaz T, Ozercan I, Elkiran T (2009) The ınhibitory effects of trastuzumab on corneal neovascularization. Am J Ophthalmol 147:703–708
Rozakis-Adcock M, Fernley R, Wade J, Pawson T, Bowtell D (1993) The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature (Lond) 363:83–85
Strieter RM, Kunkel SL, Elner VM, Martonyi CL, Koch AE, Polverini PJ, Elner SG (1992) Interleukin-8.A corneal factor that induces neovascularization. Am J Pathol 141:1279–1284
Hoffart L, Matonti F, Conrath J, Daniel L, Ridings B, Masson GS, Chavane F (2010) Inhibition of corneal neovascularization after alkali burn: comparison of different doses of bevacizumab in monotherapy or associated with dexamethasone. Clin Exp Ophthalmol 38(4):346–352
Acknowledgement
This study was supported by Scientific Research Fund of Dicle University. The authors have no financial interest in any of the products mentioned in the manuscript. We would like to thank English Lecturer Ibrahim Tunik for his checking the manuscript in terms of its grammar and spelling errors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tunik, S., Nergiz, Y., Keklikci, U. et al. The subconjunctival use of cetuximab and bevacizumab in inhibition of corneal angiogenesis. Graefes Arch Clin Exp Ophthalmol 250, 1161–1167 (2012). https://doi.org/10.1007/s00417-012-2008-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-012-2008-6