Abstract
Background
Myopic maculopathy is the leading cause of subfoveal choroidal neovascularization (CNV) in patients under 50 years of age. New antiangiogenic drugs are being used off-label with varied therapeutic schedules to treat CNV. The aim of this study is to report the anatomical and visual outcomes of myopic choroidal neovascularization (CNV) treated by two different schedules with intravitreal bevacizumab.
Methods
Prospective, comparative, consecutive, non-randomized, multicentric, interventional pilot study. Two groups of highly myopic patients with subfoveal and juxtafoveal CNV were treated by monthly intravitreal injections with 1.25 mg bevacizumab. Group 1 comprised 19 eyes treated by three consecutive monthly intravitreal injections. Group 2 comprised 20 eyes treated by one single intravitreal injection. Patients were evaluated for best-corrected visual acuity (BCVA) and optical coherence tomography (OCT) at baseline and then monthly. Fluorescein angiography was performed at baseline and when CNV activity was suspected. Further intravitreal injections were performed if CNV activity was detected.
Results
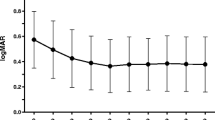
Both groups were matched for age, spherical equivalent, LogMAR BCVA, and central foveal thickness (CFT) as determined by OCT at baseline and number of eyes with previous PDT treatment. The average number of letters gained was 6.3 in group 1 vs 7.2 in group 2 (p = 0.001 and 0.09 respectively, Student's t-test for paired data). Changes in OCT were not significant for either group by the end of follow-up. The mean number injections performed was 3.2 in group 1 vs 1.7 in group 2 (p = 0.00, Mann–Whitney test). Four recurrences (four eyes) occurred in group 1 vs 15 (seven eyes) in group 2 (p = 0.001; Fisher's exact test).
Conclusions
Both schedules achieved similar results improving BCVA, though the second group required a lower number of injections, showing a higher rate of recurrences during the first year.

Similar content being viewed by others
References
Cohen SY, Laroche A, Leguen Y, Soubrane G, Coscas GJ (1996) Etiology of choroidal neovascularization in young patients. Ophthalmology 103:1241–1244
Ruiz-Moreno JM, Montero JA (2002) Long-term visual acuity after argon green laser photocoagulation of juxtafoveal choroidal neovascularization in highly myopic eyes. Eur J Ophthalmol 12:117–122
Secretan M, Kuhn D, Soubrane G, Coscas G (1997) Long-term visual outcome of choroidal neovascularization in pathologic myopia: natural history and laser treatment. Eur J Ophthalmol 7:307–316
Cekic O, Ohji M, Fujikado T, Fang XY, Hayashi A, Kusaka S, Tano Y (2000) Foveal translocation surgery and myopic subfoveal CNV membrane. Ophthalmology 107:2117
Uemura A, Thomas M (2000) Subretinal surgery for choroidal neovascularization in patients with high myopia. Arch Ophthalmol 118:344–350
Verteporfin in Photodynamic Therapy Study Group (2001) Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin. 1-year results of a randomized clinical trial--VIP report no. 1. Ophthalmology 108:841-852
Montero JA, Ruiz-Moreno JM (2003) Verteporfin photodynamic therapy in highly myopic subfoveal choroidal neovascularisation. Br J Ophthalmol 87:173–176
Ruiz-Moreno JM, Montero JA (2003) Subretinal fibrosis after photodynamic therapy in subfoveal choroidal neovascularisation in highly myopic eyes. Br J Ophthalmol 87:856–859
Degenring RF, Jonas JB (2005) Photodynamic therapy in combination with intravitreal triamcinolone for myopic choroidal neovascularization. Acta Ophthalmol Scand 83:621
Marticorena J, Gomez-Ulla F, Fernandez M, Pazos B, Rodriguez-Cid MJ, Sanchez-Salorio M (2006) Combined photodynamic therapy and intravitreal triamcinolone acetonide for the treatment of myopic subfoveal choroidal neovascularization. Am J Ophthalmol 142:335–337
Montero JA, Ruiz-Moreno JM (2007) Combined photodynamic therapy and intravitreal triamcinolone injection for the treatment of choroidal neovascularisation secondary to pathological myopia: a pilot study. Br J Ophthalmol 91:131–133
Silva RM, Ruiz-Moreno JM, Nascimento J, Carneiro A, Rosa P, Barbosa A et al (2008) Short-term efficacy and safety of intravitreal ranibizumab for myopic choroidal neovascularization. Retina 28:1117–1123
Sakaguchi H, Ikuno Y, Gomi F, Kamei M, Sawa M, Tsujikawa M, Oshima Y, Kusaka S, Tano Y (2007) Intravitreal injection of bevacizumab for choroidal neovascularisation associated with pathological myopia. Br J Ophthalmol 91:161–165
Tewari A, Dhalla MS, Apte RS (2006) Intravitreal bevacizumab for treatment of choroidal neovascularization in pathologic myopia. Retina 26:1093–1094
Yamamoto I, Rogers AH, Reichel E, Yates PA, Duker JS (2007) Intravitreal bevacizumab (Avastin) as treatment for subfoveal choroidal neovascularisation secondary to pathological myopia. Br J Ophthalmol 91:157–160
Ruiz-Moreno JM, Montero JA, Gomez-Ulla F, Ares S (2009) Intravitreal bevacizumab to treat subfoveal choroidal neovascularisation in highly myopic eyes: 1-year outcome. Br J Ophthalmol 93:448–451
Mandal S, Venkatesh P, Sampangi R, Garg S (2007) Intravitreal bevacizumab (Avastin) as primary treatment for myopic choroidal neovascularization. Eur J Ophthalmol 17:620–626
Hernandez-Rojas ML, Quiroz-Mercado H, Dalma-Weiszhausz J, Fromow-Guerra J, Amaya-Espinosa A, Solis-Vivanco A, Reyna-Castelan E, Abraham-Marin M, Martinez-Castellanos MA, Aiello LP (2007) Short-term effects of intravitreal bevacizumab for subfoveal choroidal neovascularization in pathologic myopia. Retina 27:707–712
Chan WM, Lai TY, Liu DT, Lam DS (2007) Intravitreal bevacizumab (Avastin) for myopic choroidal neovascularization: six-month results of a prospective pilot study. Ophthalmology 114:2190–2196
Ruiz-Moreno JM, Gomez-Ulla F, Montero JA, Ares S, Lopez-Lopez F, Rodriguez M, Fernandez M (2009) Intravitreous bevacizumab to treat subfoveal choroidal neovascularization in highly myopic eyes: short-term results. Eye 23:334–338
Chan WM, Lai TY, Liu DT, Lam DS (2009) Intravitreal bevacizumab (Avastin) for myopic choroidal neovascularisation: 1-year results of a prospective pilot study. Br J Ophthalmol 93:150–154
Gharbiya M, Allievi F, Mazzeo L, Gabrieli CB (2009) Intravitreal bevacizumab treatment for choroidal neovascularization in pathologic myopia: 12-month results. Am J Ophthalmol 147(84–93):e81
Ikuno Y, Sayanagi K, Soga K, Sawa M, Tsujikawa M, Gomi F, Tano Y (2009) Intravitreal bevacizumab for choroidal neovascularization attributable to pathological myopia: one-year results. Am J Ophthalmol 147(94–100):e101
Wu PC, Chen YJ (2009) Intravitreal injection of bevacizumab for myopic choroidal neovascularization: 1-year follow-up. Eye 23(11):2042–2045
Arias L, Caminal JM, Casas L, Masuet C, Badia MB, Rubio M, Pujol O, Arruga J (2008) A study comparing two protocols of treatment with intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Br J Ophthalmol 92:1636–1641
Garcia-Layana A, Salinas-Alaman A, Maldonado MJ, Sainz-Gomez C, Fernandez-Hortelano A (2006) Optical coherence tomography to monitor photodynamic therapy in pathological myopia. Br J Ophthalmol 90:555–558
Laud K, Spaide RF, Freund KB, Slakter J, Klancnik JM Jr (2006) Treatment of choroidal neovascularization in pathologic myopia with intravitreal bevacizumab. Retina 26:960–963
Nguyen QD, Shah S, Tatlipinar S, Do DV, Anden EV, Campochiaro PA (2005) Bevacizumab suppresses choroidal neovascularisation caused by pathological myopia. Br J Ophthalmol 89:1368–1370
Acknowledgments
This study has been supported in part by a grant of the Spanish Ministry of Health, Instituto de Salud Carlos III, Red Temática de Investigación Cooperativa en Salud "Patología ocular del envejecimiento, calidad visual y calidad de vida" (RD07/0062/0019).
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have full control of all primary data, and they agree to allow Graefe’s Archive for Clinical and Experimental Ophthalmology to review their data if requested.
The design of this study was reviewed and approved by the clinical ethics committee at Vissum Ophthalmological Institute of Alicante.
Written informed consent and individualized approval from the National Ministry of Health was obtained prior to the procedure.
This study has been performed in accordance with the ethical standards of the 1964 Declaration of Helsinki, and data gathering was performed after obtaining written informed consent. Patients were informed about the off-label situation of this therapy and women of child bearing age were also informed about the possible risks of pregnancy and in utero exposition.
Rights and permissions
About this article
Cite this article
Ruiz-Moreno, J.M., Montero, J.A. & Amat-Peral, P. Myopic choroidal neovascularization treated by intravitreal bevacizumab: comparison of two different initial doses. Graefes Arch Clin Exp Ophthalmol 249, 595–599 (2011). https://doi.org/10.1007/s00417-010-1599-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-010-1599-z