Abstract
Background
Mutations in the gene CHN1 have been described in autosomal dominant Duane’s retraction syndrome (DRS) and mutations have been shown to interfere with normal innervation of target eye muscles by oculomotor axons in chick embryos. We screened for CHN1 mutations in patients with various congenital ocular motility disorders.
Methods
Altogether, 29 patients with different congenital ocular motility disorders and a positive family history of congenital ocular motility disturbances or strabismus or bilateral affection or accompanying congenital disorders were enrolled in this study. DNA samples of patients suffering from DRS (n = 5), Brown syndrome (n = 13), other congenital motility disorders of the oblique eye muscles (n = 6), double elevator palsy (n = 4), and vertical retraction syndrome (n = 1) were investigated by direct sequencing of all coding exons of CHN1.
Results
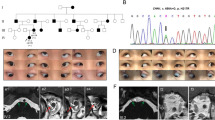
In the families of our index patients with DRS, other family members displayed DRS, see-saw nystagmus, infantile esotropia, microtropia, or Brown syndrome, respectively. In the families of our patients with cases of Brown syndrome, bilateral abduction deficiency, infantile esotropia, and unspecified strabismus occurred. The patients with congenital disorders of the oblique muscles and with congenital elevation deficiencies other than Brown syndrome had relatives with ptosis, infantile esotropia, DRS, congenital abduction deficiency, and unspecified forms of strabismus. Thus a considerable intrafamilial overlap between different types of congenital forms of motility disorders and strabismus does exist. No mutations were detected in the CHN1 gene in our patients. In addition to known polymorphisms, we identified four novel heterozygous single-nucleotide substitutions, one in the 5′UTR, two in intronic regions, and one in the coding region leading to a synonymous amino acid substitution.
Conclusions
We found no evidence for a causative involvement of CHN1 mutations in congenital ocular motor anomalies different from autosomal dominant Duane’s retraction syndrome and provide further evidence for genetic heterogeneity in familial forms of DRS.




Similar content being viewed by others
References
Miyake N, Chilton J, Psatha M, Cheng L, Andrews C, Chan WM, Law K, Crosier M, Lindsay S, Cheung M, Allen J, Gutowski NJ, Ellard S, Young E, Iannaccone A, Appukuttan B, Stout JT, Christiansen S, Ciccarelli ML, Baldi A, Campioni M, Zenteno JC, Davenport D, Mariani LE, Sahin M, Guthrie S, Engle EC (2008) Human CHN1 mutations hyperactivate alpha2-chimaerin and cause Duane’s retraction syndrome. Science 321:839–843
Gutowski NJ, Bosley TM, Engle EC (2003) 110th ENMC International Workshop: the congenital cranial dysinnervation disorders (CCDDs). Naarden, The Netherlands, 25–27 October, 2002. Neuromuscul Disord 13:573–578
Hotchkiss MG, Miller NR, Clark AW, Green WR (1980) Bilateral Duane’s retraction syndrome. A clinical-pathologic case report. Arch Ophthalmol 98:870–874
Huber A, Esslen E (1969) Duane’s syndrome; observations on the pathogenesis and etiology of different forms of the Stilling-Duane-Turk retraction syndrome. Doc Ophthalmol 26:619–628
Engle EC (2007) Genetic basis of congenital strabismus. Arch Ophthalmol 125:189–195
Engle EC (2007) Oculomotility disorders arising from disruptions in brainstem motor neuron development. Arch Neurol 64:633–637
Traboulsi EI (2004) Congenital abnormalities of cranial nerve development: overview, molecular mechanisms, and further evidence of heterogeneity and complexity of syndromes with congenital limitation of eye movements. Trans Am Ophthalmol Soc 102:373–389
Ozkurt H, Basak M, Oral Y, Ozkurt Y (2003) Magnetic resonance imaging in Duane’s retraction syndrome. J Pediatr Ophthalmol Strabismus 40:19–22
Murillo-Correa CE, Kon-Jara V, Engle EC, Zenteno JC (2009) Clinical features associated with an I126M alpha2-chimaerin mutation in a family with autosomal-dominant Duane retraction syndrome. J AAPOS 13:245–248
Bosley TM, Salih MA, Alorainy IA, Oystreck DT, Nester M, Abu-Amero KK, Tischfield MA, Engle EC (2007) Clinical characterization of the HOXA1 syndrome BSAS variant. Neurology 69:1245–1253
Bosley TM, Alorainy IA, Salih MA, Aldhalaan HM, Abu-Amero KK, Oystreck DT, Tischfield MA, Engle EC, Erickson RP (2008) The clinical spectrum of homozygous HOXA1 mutations. Am J Med Genet A 146A:1235–1240
Chan WM, Traboulsi EI, Arthur B, Friedman N, Andrews C, Engle EC (2006) Horizontal gaze palsy with progressive scoliosis can result from compound heterozygous mutations in ROBO3. J Med Genet 43:e11
Holve S, Friedman B, Hoyme HE, Tarby TJ, Johnstone SJ, Erickson RP, Clericuzio CL, Cunniff C (2003) Athabascan brainstem dysgenesis syndrome. Am J Med Genet A 120A:169–173
Jen JC, Chan WM, Bosley TM, Wan J, Carr JR, Rub U, Shattuck D, Salamon G, Kudo LC, Ou J, Lin DD, Salih MA, Kansu T, Al Dhalaan H, Al Zayed Z, MacDonald DB, Stigsby B, Plaitakis A, Dretakis EK, Gottlob I, Pieh C, Traboulsi EI, Wang Q, Wang L, Andrews C, Yamada K, Demer JL, Karim S, Alger JR, Geschwind DH, Deller T, Sicotte NL, Nelson SF, Baloh RW, Engle EC (2004) Mutations in a human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesis. Science 304:1509–1513
Nakano M, Yamada K, Fain J, Sener EC, Selleck CJ, Awad AH, Zwaan J, Mullaney PB, Bosley TM, Engle EC (2001) Homozygous mutations in ARIX(PHOX2A) result in congenital fibrosis of the extraocular muscles type 2. Nat Genet 29:315–320
Tischfield MA, Bosley TM, Salih MA, Alorainy IA, Sener EC, Nester MJ, Oystreck DT, Chan WM, Andrews C, Erickson RP, Engle EC (2005) Homozygous HOXA1 mutations disrupt human brainstem, inner ear, cardiovascular and cognitive development. Nat Genet 37:1035–1037
Yamada K, Hunter DG, Andrews C, Engle EC (2005) A novel KIF21A mutation in a patient with congenital fibrosis of the extraocular muscles and Marcus Gunn jaw-winking phenomenon. Arch Ophthalmol 123:1254–1259
Yamada K, Chan WM, Andrews C, Bosley TM, Sener EC, Zwaan JT, Mullaney PB, Ozturk BT, Akarsu AN, Sabol LJ, Demer JL, Sullivan TJ, Gottlob I, Roggenkaemper P, Mackey DA, De Uzcategui CE, Uzcategui N, Ben-Zeev B, Traboulsi EI, Magli A, de Berardinis T, Gagliardi V, Awasthi-Patney S, Vogel MC, Rizzo JF 3rd, Engle EC (2004) Identification of KIF21A mutations as a rare cause of congenital fibrosis of the extraocular muscles type 3 (CFEOM3). Investig Ophthalmol Vis Sci 45:2218–2223
Yazdani A, Chung DC, Abbaszadegan MR, Al-Khayer K, Chan WM, Yazdani M, Ghodsi K, Engle EC, Traboulsi EI (2003) A novel PHOX2A/ARIX mutation in an Iranian family with congenital fibrosis of extraocular muscles type 2 (CFEOM2). Am J Ophthalmol 136:861–865
Kohlhase J, Heinrich M, Schubert L, Liebers M, Kispert A, Laccone F, Turnpenny P, Winter RM, Reardon W (2002) Okihiro syndrome is caused by SALL4 mutations. Hum Mol Genet 11:2979–2987
Tischfield MA, Baris HN, Wu C, Rudolph G, Van Maldergem L, He W, Chan WM, Andrews C, Demer JL, Robertson RL, Mackey DA, Ruddle JB, Bird TD, Gottlob I, Pieh C, Traboulsi EI, Pomeroy SL, Hunter DG, Soul JS, Newlin A, Sabol LJ, Doherty EJ, de Uzcategui CE, de Uzcategui N, Collins ML, Sener EC, Wabbels B, Hellebrand H, Meitinger T, de Berardinis T, Magli A, Schiavi C, Pastore-Trossello M, Koc F, Wong AM, Levin AV, Geraghty MT, Descartes M, Flaherty M, Jamieson RV, Moller HU, Meuthen I, Callen DF, Kerwin J, Lindsay S, Meindl A, Gupta ML Jr, Pellman D, Engle EC (2010) Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell 140:74–87
Neugebauer A, Fricke J (2010) Congenital cranial dysinnervation disorders: facts and perspectives to understand ocular motility disorders. In: Lorenz B, Brodsky M (eds) Pediatric ophthalmology, neuroophthalmology, genetics. Springer, Berlin Heidelberg New York, pp 77–94
Connell BJ, Wilkinson RM, Barbour JM, Scotter LW, Poulsen JL, Wirth MG, Essex RW, Savarirayan R, Mackey DA (2004) Are Duane syndrome and infantile esotropia allelic? Ophthalmic Genet 25:189–198
Wilson ME, Eustis HS Jr, Parks MM (1989) Brown’s syndrome. Surv Ophthalmol 34:153–172
Papst W, Stein HJ (1969) Etiology of the superior oblique tendon sheath syndrome. Klin Monbl Augenheilkd 154:506–518
Stein HJ, Papst W (1969) Electromyographic studies on the pathogenesis and therapy of the superior oblique tendon sheath syndrome (Brown’s syndrome). Ber Zusammenkunft Dtsch Ophthalmol Ges 69:618–624
Calabrese G, Stuppia L, Morizio E, Guanciali Franchi P, Pompetti F, Mingarelli R, Marsilio T, Rocchi M, Gallenga PE, Palka G, Dallapiccola B (1998) Detection of an insertion deletion of region 8q13-q21.2 in a patient with Duane syndrome: implications for mapping and cloning a Duane gene. Eur J Hum Genet 6:187–193
Vincent C, Kalatzis V, Compain S, Levilliers J, Slim R, Graia F, Pereira ML, Nivelon A, Croquette MF, Lacombe D, Vigneron J, Helias J, Broyer M, Callen DF, Haan EA, Weissenbach J, Lacroix B, Bellané-Chantelot C, Le Paslier D, Cohen D, Petit C (1994) A proposed new contiguous gene syndrome on 8q consists of Branchio-Oto-Renal (BOR) syndrome, Duane syndrome, a dominant form of hydrocephalus and trapeze aplasia; implications for the mapping of the BOR gene. Hum Mol Genet 3:1859–1866
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Volk, A.E., Fricke, J., Strobl, J. et al. Analysis of the CHN1 gene in patients with various types of congenital ocular motility disorders. Graefes Arch Clin Exp Ophthalmol 248, 1351–1357 (2010). https://doi.org/10.1007/s00417-010-1417-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-010-1417-7