Abstract
Purpose
To evaluate the efficacy and safety of topical cyclosporine A 2% in the prevention of graft rejection in high-risk keratoplasty.
Methods
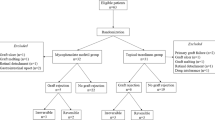
A randomized clinical trial was conducted in which penetrating keratoplasty was performed in 78 eyes that were at high risk for keratoplasty. The study group (n = 39) received topical cyclosporine A 2% drops and control group (n = 39) received polyvinyl alcohol 1.4% drops. In addition, both groups received corticosteroid eye drops after surgery. The main outcome measures were rejection-free interval and reversal of graft rejection.
Results
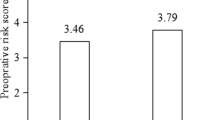
The most common indication for penetrating keratoplasty was failed previous graft in both groups. The best corrected visual acuity at the end of 1 year was 0.31 ± 0.18 in the study group and 0.24 ± 0.17 in the control group (p = 0.14). Seven patients in each group had one episode of graft rejection and one patient in the control group had two episodes of graft rejection. The mean duration after which the patients developed graft rejection after keratoplasty was 7.92 ± 1.45 months and 6.50 ± 2.72 months in the study and control group, respectively (p = 0.20). Six patients showed complete reversal of rejection in the study group and four patients showed reversal in the control group (p = 0.03).
Conclusions
Topical cyclosporine A 2% eye drops do not prevent occurrence of graft rejection in high-risk keratoplasty. However, the eyes receiving topical cyclosporine stand a better chance of reversal of the episode of graft rejection.

Similar content being viewed by others
References
Williams KA, Lowe MT, Bartlett CM, Kelly L, Coster DJ (2007) The Australian Corneal Graft Registry 2007 Report. Flinders University Press, Adelaide
Wolfe RA (2004) Long-term renal allograft survival: a cup half-full and half-empty. Am J Transplant; 4:1215–1216
Wilson SE, Kaufman HE (1990) Graft failure after penetrating keratoplasty. Surv Ophthalmol; 34:325–356
The Collaborative Corneal Transplantation Research Group. The collaborative corneal transplantation studies (CCTS) (1992) Effectiveness of histocompatibility matching in high risk corneal transplantation. Arch Ophthalmol 110:1392–1403
Khodadaust AA (1973) The allograft rejection reaction: the leading cause of late graft failure of clinical corneal grafts. In: Porter R, Knight J (eds) Corneal graft failure. Ciba Foundation Symposium 15. Elsevier, Amsterdam, pp 151–164
Barba KR, Samy A, Lai C, Perlman JI, Bouchard CS (2000) Effect of topical anti-inflammatory drugs on corneal and limbal wound healing. J Cataract Refract Surg 26:893–897
Leibowitz H (1979) Ocular toxicity of corticosteroids. In: Leopold IH, Burns RP (eds) Symposium on ocular therapy, vol. 11, Wiley, New York, pp 108–109
Armaly MF (1963) Effect of corticosteroids on intraocular pressure and fluid dynamics. I. The effect of dexamethasone in the normal eye. Arch Ophthalmol 70:482–491
Lewis JM, Priddy T, Judd J, Gordon MO, Kass MA, Kolker AE, Becker (1988) Intraocular pressure response to topical dexamethasone as a predictor for the development of primary open-angle glaucoma. Am J Ophthalmol 106:607–612
Becker B (1965) Intraocular pressure response to topical corticosteroids. Invest Ophthalmol 4:198–205
Belin MW, Bouchard CS, Phillips TM (1990) Update on topical cyclosporine A. Background, immunology, and pharmacology. Cornea 9:184–195
Hill JC (1995) Systemic cyclosporine in high-risk keratoplasty: long-term results. Eye 9:422–428
Poon AC, Forbes JE, Dart JK, Subramaniam S, Bunce C, Madison P, Ficker LA, Tuft SJ, Gartry DS, Buckley RJ (2001) Systemic cyclosporine A in high-risk penetrating keratoplasties: a case-control study. Br J Ophthalmol 85:1464–1469
Tandon R, Chawla B, Verma K, Sharma N, Titiyal JS (2008) Outcome of treatment of Mooren ulcer with topical cyclosporine A 2%. Cornea 27:859–861
Zhao JC, Jin XY (1995) Local therapy of corneal allograft rejection with cyclosporine. Am J Ophthalmol 119:189–194
Cosar CB, Laibson PR, Cohen EJ, Rapuano CJ (2003) Topical cyclosporine in pediatric keratoplasty. Eye Contact Lens 29:103–107
Wiederholt M, Kossendrup D, Schulz W, Hoffmann F (1986) Pharmacokinetic of topical cyclosporine A in the rabbit eye. Invest Ophthalmol Vis Sci 27:519–524
Inoue K, Amano S, Kimura C, Sato T, Fujita N, Kagaya F, Kaji Y, Oshika T, Tsuru T, Araie M (2000) Long-term effects of topical cyclosporine A treatment after penetrating keratoplasty. Jpn J Ophthalmol 44:302–305
Price MO, Price FW (2006) Efficacy of topical cyclosporine 0.05% for prevention of cornea transplant rejection episode. Ophthalmology 113:1785–1790
Poon A, Constantinou M, Lamoureux E, Taylor HR (2008) Topical cyclosporine A in the treatment of acute graft rejection: a randomized controlled trial. Clin Experiment Ophthalmol 36:415–421
Acknowledgements
We thank the Indian Council of Medical Research, New Delhi, for the approval of the study protocol.
Financial interests
None
Proprietary interests
None
Competing interests
None
Funding
The study was funded by a financial grant from the Indian Council of Medical Research, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Clinical Trial Registration: http://www.controlled-trials.com/ISRCTN52781697: ISRCTN52781697
Rights and permissions
About this article
Cite this article
Sinha, R., Jhanji, V., Verma, K. et al. Efficacy of topical cyclosporine A 2% in prevention of graft rejection in high-risk keratoplasty: a randomized controlled trial. Graefes Arch Clin Exp Ophthalmol 248, 1167–1172 (2010). https://doi.org/10.1007/s00417-010-1388-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-010-1388-8