Abstract
Background
In order to understand the role of ocular blood flow in normal and pathological conditions, knowledge of the pharmacological control mechanisms involved in the ocular vascular bed is essential. The present study was designed to investigate the reactivity of the rabbit external ophthalmic artery and its collaterals to amlodipine, in order to answer two questions: (1) What are amlodipine effects upon perfusion pressure and spontaneous oscillations in the in situ perfused rabbit eyes? (2) Can intraarterial amlodipine counteract ET-1 induced vasoconstriction?
Methods
Rabbit external ophthalmic arteries (n = 12) in a head-mounted preparation were cannulated and perfused with warmed tyrode. Vasomotor response curves to intraarterial injections of amlodipine 3 mg/ml followed by phenylephrine 250 µg (group A, n = 6) and to amlodipine 3 mg/ml after an intraarterial injection of endothelin-1 (ET-1) 27 µg/ml (group B, n = 6) were obtained. For statistical analysis, the paired t-test and Fourier analysis of frequency spectrums of spontaneous oscillations were used.
Results
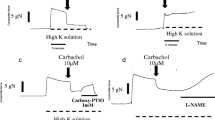
Before any drug administration, spontaneous oscillations were observed in the 12 rabbit models. In group A, amlodipine elicited vasodilation and a decrease in frequency and amplitude of the oscillations. In group B, ET-1 induced an increase in vasoconstrictor tone and vasomotion became more evident. With amlodipine after ET-1, we obtained vasodilation and abolition of the vasospasm.
Conclusions
Our study has two main conclusions: (1) amlodipine, an L-type calcium channel blocker, caused intense vasodilation and decreased both frequency and amplitude of the spontaneous oscillations observed in the rabbit external ophthalmic artery and its collaterals, and (2) when we applied amlodipine in arteries previously contracted by the administration of ET-1, vascular resistance greatly decreased and spontaneous oscillations were abolished. Since ET-1 levels are increased in several ischemic ocular diseases, amlodipine might be beneficial in these patients, allowing a protective action against vasospasm.







Similar content being viewed by others
References
Cooke K, Snyder P (1998) Calcium channel blockers in veterinary medicine. J Vet Intern Med 12:123–131
Haefliger IO, Meyer P, Flammer J, Lüscher T (1994) The vascular endothelium as a regulator of the ocular circulation. A new concept in ophthalmology? Surv Ophthalmol 39(2):123–132
Sugiyama T, Moriya S, Oku H (1995) Association of endothelin-1 with normal tension glaucoma: clinical and fundamental studies. Surv Ophthalmol 39:S49–S56
Evans D, Harris A, Garret M (1999) Glaucoma patients demonstrate faulty autoregulation of ocular blood flow during posture change. Brit J Ophthalmol 83:809–813
Källberg M, Brooks D, Komaromy A, Miyabayashi T, Bradshaw P (2003) The effect of an L-type calcium channel blocker on the hemodynamics of orbital arteries in dogs. Vet Ophthalmol 6(2):141–146
Delgado E, Marques-Neves C, Rocha I, Sales-Luis J, Silva-Carvalho L (2005) Adrenergic reactivity and myogenic tone in a perfused model of isolated rabbit eye. Vet Ophthalmol 8(6):431
Delgado E, Marques-Neves C, Rocha I, Sales-Luis J, Silva-Carvalho L (2009) Intrinsic vasomotricity and adrenergic effects in a model of isolated rabbit eye. Acta Ophthalmol 87:443–449
Hoste A, Boels P, Brutsaert D, De Laey J (1989) Effect of alpha-1 and beta agonists on contraction of bovine retinal resistance arteries in vitro. Invest Ophthalmol Vis Sci 30:44–50
Nyborg N, Korsgaard N, Nielsen P (1990) Active wall tension-length curve and morphology of isolated bovine retinal small arteries: important feature for pharmacodynamic studies. Invest Ophthalmol Vis Sci 51:217–224
Yu D, Alder V, Cringle S, Su E, Yu P (1994) Vasoactivity of intraluminal and extraluminal agonists in perfused retinal arteries. Invest Ophthalmol Vis Sci 35:4087–4099
Jeppesen P, Aalkjaer C, Bek T (2003) Myogenic response in isolated porcine retinal arteríoles. Curr Eye Res 27(4):217–222
Hessellund A, Jeppesen P, Aalkjaer C, Bek T (2003) Characterization of vasomotion in porcine retinal arterioles. Acta Ophthalmol Scand 81(3):278–282
Delaey C, Van der Voorde J (2000) Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res 32(6):249–256
Delgado E, Marques-Neves C, Rocha I, Sales-Luis J, Silva-Carvalho L (2007) Effects of amlodipine in an experimental model of isolated rabbit eye. ARVO Congress, Florida, EUA, Abstract nº 2289
Castro-Correia J (1996) Understanding the choroid. Int Ophthalmol 19:135–147
Kiel J, Shepperd A (1992) Autoregulation of choroidal blood flow in the rabbit. Invest Ophthalmol Vis Sci 33(8):2399–2410
Kiel J, van Heuven J (1995) Ocular perfusion pressure and choroidal blood flow in the rabbit. Invest Ophthalmol Vis Sci 36(3):579–585
Kiel J (1999) Modulation of choroidal autoregulation in the rabbit. Exp Eye Res 69(4):413–429
Delgado E, Marques-Neves C, Rocha I, Sales-Luis J, Silva-Carvalho L (2009) The influence of single-injection of endothelin-1 on spontaneous intravascular pressure oscillations in choroidal arterioles in vitro. Acta Ophthalmol [epub ahead of print], doi:10.1111.j.1755.3768.2009.01537.x
Meyer P, Flammer J, Lüscher T (1992) Endothelium-dependent regulation of the ophthalmic microcirculation in the porcine eye: role of nitric oxide and endothelins. Invest Ophthalmol Vis Sci 34(13):3614–3621
Jeppesen P, Sanye-Hajari J, Bek T (2007) Increased blood pressure induces a diamenter response of retinal arterioles that increases with decreasing arteriolar diameter. Invest Ophthalmol Vis Sci 48(1):328–331
Holmgaard K, Aalkjaer C, Lambert JD, Hessellund A, Bek T (2008) The relaxing effect of perivascular tissue on porcine retinal arterioles in vitro is mimicked by N-methyl-D-aspartate and is blocked by prostaglandin synthesis inhibition. Acta Ophthalmol 86(1):26–33
Harino S, Riva C, Petrig B (1992) Intravenous nicardipine in cats increases optic nerve head but not retinal blood flow. Invest Ophthalmol Vis Sci 33:2885–2890
Tamaki Y, Araie M, Tomita K (1999) Effects of pranidipine, a new calcium antagonist, on circulation in the choroids, retina and optic nerve head. Curr Eye Res 19:241–247
Netland P, Chaturvedi N, Dreyer E (1993) Calcium channel blockers in the management of low-tension and open-angle glaucoma. Am J Physiol 115:608–613
Wilson R, Chang W, Sergott R (1997) A colour Doppler analysis of nifedipine-induced posterior ocular blood flow changes in open-angle glaucoma. J Glaucoma 6:231–236
Yamamoto T, Niwa Y, Kawakami H (1998) The effect of nilvadipine, a calcium-channel blocker, on the hemodynamics of retrobulbar vessels in normal-tension glaucoma. J Glaucoma 7:301–305
Källberg M, Brooks D, Garcia-Sanchez G (2000) Endothelin-1 levels in the aqueous humour of dogs with glaucoma. Invest Ophthalmol Vis Sci 41:S255
Kaiser HJ, Flammer J, Wenk M (1995) Endothelin-1 plasma levels in normal tension glaucoma: abnormal responses to postural changes. Graefes Arch Clin Exp Ophthalmol 233:484–488
Gasser P (1999) Why study vascular factors in glaucoma? Int Ophthalmol 22:221–225
Gasser P, Flammer J (1990) Short and long term effect of nifedipine on the visual field in patients with presumed vasospasm. J Int Med Res 18:334–339
Jeppesen P, Knudsen S, Poulsen P, Mogensen C, Schmitz O, Bek T (2007) Response of retinal arteriole diameter to increased blood pressure during acute hyperglycaemia. Acta Ophthalmol Scand 85(3):280–286
Author information
Authors and Affiliations
Corresponding author
Additional information
Luís Silva-Carvalho—in memoriam
This work was supported by CIISA (Centro de Investigação Interdisciplinar em Sanidade Animal). There are no financial interests in the equipment or methods described. The authors have full control of all primary data, and they agree to allow Graefe's Archive for Clinical and Experimental Ophthalmology to review our data.
Rights and permissions
About this article
Cite this article
Delgado, E., Marques-Neves, C., Rocha, I. et al. Amlodipine effects on vasomotion in rabbit external ophthalmic artery. Graefes Arch Clin Exp Ophthalmol 248, 213–221 (2010). https://doi.org/10.1007/s00417-009-1235-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-009-1235-y