Abstract
Purpose
The purpose of this study is to evaluate the retinal toxicity of two doses of adalimumab (Humira), a recombinant human IgG1 monoclonal antibody specific for human tumor necrosis factor (TNF), when injected intravitreally in rabbits.
Methods
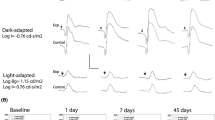
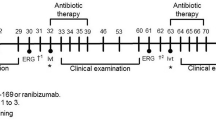
Sixteen male pigmented rabbits (divided into two groups, eight animals per group) were used for this study. Two concentrations of adalimumab were tested: 0.5 mg/0.1 ml and 5 mg/0.1 ml. Each concentration was injected intravitreally randomly in one eye (study group) of each rabbit (group I received 0.5 mg/0.1 ml and group II received 5.0 mg/0.1 ml), while in the other eye (control group) 0.1 ml of sterile balanced saline solution (BSS) was injected. Slit-lamp and funduscopic examinations were performed every second day for 2 weeks for signs of infection, inflammation and toxicity. A baseline electroretinogram (ERG) was performed before the experiment and at the last follow-up day (day 14). ERG examination followed ISCEV standards. At the last follow-up day, the animals were sacrificed and the enucleated eyes were prepared for histological evaluation of retinal toxicity.
Results
No differences in ERG responses at photopic and scotopic conditions were observed in eyes injected with either concentration of adalimumab or BSS. Furthermore, histologic examination of the retina in the enucleated eyes (in all groups) did not demonstrate any evidence of drug toxicity.
Conclusions
Intravitreal adalimumab did not appear toxic to the retina in this experimental model at concentrations of 0.5 and 5 mg. If found safe in additional studies, intravitreally injected adalimumab could be evaluated for efficacy in the treatment of inflammatory eye conditions.


Similar content being viewed by others
References
Theodossiadis PG, Markomichelakis NN, Sfikakis PP (2007) Tumor necrosis factor antagonists: preliminary evidence for an emerging approach in the treatment of ocular inflammation. Retina 27(4):399–413, doi:10.1097/MAJ.0b013e3180318fbc
Santos Lacomba M, Marcos Martín C, Gallardo Galera JM, Gómez Vidal MA, Collantes Estévez E, Ramírez Chamond R, Omar MM (2001) Aqueous humor and serum tumor necrosis factor-a in clinical uveitis. Ophthalmic Res 33:251–255, doi:10.1159/000055677
Palexas GN, Sussman G, Welsh NH (1992) Ocular and systemic determination of IL-1 beta and tumor necrosis factor in a patient with ocular inflammation. Scand J Immunol 11(Suppl):173–175, doi:10.1111/j.1365–3083.1992.tb01645.x
Nakamura S, Yamakawa T, Sugita M, Kijima M, Ishioka M, Tanaka S, Ohno S (1994) The role of tumor necrosis factor-alpha in the induction of experimental autoimmune uveoretinitis in mice. Invest Ophthalmol Vis Sci 35:3884–3889
Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, Kollias G (1991) Transgenic mice expressing human tumor necrosis factor: a predictive genetic model of arthritis. EMBO J 10:4025–4031
Chilton F, Collet RA (2008) Treatment choices, preferences and decision-making by patients with rheumatoid arthritis. Musculoskeletal Care 6(1):1–14, doi:10.1002/msc.110
Danese S, Panago N, Angelucci E et al (2007) Tumor necrosis factor-alpha monoclonal antibodies for Crohn’s disease: tipping the balance. Curr Med Chem 14(14):1489–1497, doi:10.2174/092986707780831104
Sfikakis PP, Theodossiadis PG, Katsiari CG, Kaklamanis P, Markomichelakis NN (2001) Effect of infliximab on sight-threatening panuveitis in Behcet’s disease. Lancet 358:295–296, doi:10.1016/S0140–6736(01)05497–6
Reiff A, Takei S, Sadeghi S (2001) Etanercept therapy in children with treatment-resistant uveitis. Arthritis Rheum 45:1411–1415 doi:10.1002/1529–0131(200106)44:6<1411::AID-ART235>3.0.CO;2-O
Markomichelakis NN, Theodossiadis PG, Pantelia E, Papaefthimiou S, Theodossiadis GP, Sfikakis PP (2004) Infliximab for chronic cystoid macular edema associated with uveitis. Am J Ophthalmol 138:648–650, doi:10.1016/j.ajo.2004.04.066
Nakazawa T, Nakazawa C, Matsubara A, Noda K, Hisatomi T, She H, Michaud N, Hafezi-Moghadam A, Miller JW, Benowitz LI (2006) Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J Neurosci 26(49):12633–12641, doi:10.1523/JNEUROSCI.2801–06.2006
Chen YF, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, Fry-Smith A, Burls A (2006) A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and economic evaluation of their cost-effectiveness. Health Technol Assess. 10(42):iii-iv, xi-xiii, 1–229
McLeod C, Bagust A, Boland A, Dagenais P, Dickson R, Dundar Y, Hill RA, Jones A, Mujica Mota R, Walley T (2007) Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation. Health Technol Assess 11(28):1–158
Ehlers S (2005) Why does tumor necrosis factor targeted therapy reactivate tuberculosis? J Rheumatol Suppl 74:35–39
Mukhopadhyay S, Hoidal JR, Mukherjee TK (2006) Role of TNF-alpha in pulmonary pathophysiology. Respir Res 7:125, doi:10.1186/1465–9921–7–125
Lee JH, Slifman NR, Gershon SK, Edwards ET, Schwieterman WD, Siegel JN, Wise RP, Brown SL, Udall JN Jr, Braun MM (2002) Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum 46(10):2565–2570, doi:10.1002/art.10583
Sarzi-Puttini P, Atzeni F, Shoenfeld Y, Ferraccioli G (2005) TNF-alha, rheumatoid arthritis, and heart failure: a rheumatological dilemma. Autoimmun Rev 4(3):153–161, doi:10.1016/j.autrev.2004.09.004
Selmaj K, Raine CS, Cross AH (1991) Anti-tumor necrosis factor therapy abrogates autoimmune demyelination. Ann Neurol 30(5):694–700, doi:10.1002/ana.410300510
Brown SL, Greene MH, Gershon SK, Edwards ET, Braun MM (2002) Tumor necrosis factor antagonist therapy and lymphoma development: twenty six cases reported to the Food and Drug Administration. Arthritis Rheum 46(12):3151–3158, doi:10.1002/art.10679
Lee HH, Song IH, Friedrich M, Gauliard A, Detert J, Röwert J, Audring H, Kary S, Burmester GR, Sterry W, Worm M (2007) Cutaneous side effects in patients with rheumatic diseases during application of tumor necrosis factor alpha antagonists. Br J Dermatol 156(3):486–491, doi:10.1111/j.1365–2133.2007.07682.x
Tsilimbaris MK, Panagiotoglou TD, Charisis SK, Anastasakis A, Krikonis TS, Christodoulakis E (2007) The use of intravitreal etanercept in diabetic macular edema. Semin Ophthalmol 22(2):75–79, doi:10.1080/08820530701418243
Bach M, Meigen T (1999) Do’s and don’ts in Fourier analysis of steady state potentials. Doc Ophthalmol 99(1):69–82, doi:10.1023/A:1002648202420
Peyman GA (1977) Antibiotic administration in the treatment of bacterial endophthalmitis. II. Intravitreal injections. Surv Ophthalmol 21(332):339–346
Flynn HW Jr, Pulido JS, Pflugfelder SC, Davis JL, Culbertson WW, Roussel TJ, Miller D (1991) Endophthalmitis therapy: changing antibiotic sensitivity patterns and current therapeutic recommendations. Arch Ophthalmol 109:175–176
Aydin E, Kazi AA, Peyman GA, Esfahani MR (2006) Intravitreal toxicity of moxifloxacin. Retina 26:187–190, doi:10.1097/00006982–200602000–00011
Jager RD, Aiello LP, Patel SC, Cunningham ET Jr (2004) Risks of intravitreous injections: a comprehensive review. Retina 26:676–698, doi:10.1097/00006982–200410000–00002
Goldstein M, Zemel E, Loewenstein A, Perlman I (2006) Retinal toxicity of indocyanine green in albino rabbits. Invest Ophthalmol Vis Sci 46(5):2100–2107, doi:10.1167/iovs.05–0206
Manzano RP, Peyman GA, Carvounis PE, Kivilcim M, Khan P, Chevez-Barrios P, Takahashi W (2008) Ocular toxicity of intravitreous adalimumab (Humira) in the rabbit. Graefes Arch Clin Exp Ophthalmol 246(6):907–911, doi:10.1007/s00417–008–0765-z
Fauser S, Kalbacher H, Alteheld N et al (2004) Pharmacokinetics and safety of intravitreally delivered etanercept. Graefes Arch Clin Exp Ophthalmol 242:582–586, doi:10.1007/s00417–004–0895-x
Kivilcim M, Peyman GA, Kazi AA, Dellacroce J, Ghobrial RN, Manzano R (2007) Intravitreal toxicity of high-dose etanercept. J Ocul Pharmacol Ther 23(1):57–62, doi:10.1089/jop.2006.0083
Giansanti F, Ramazzotti M, Vannozzi L, Rapizzi E, Fiore T, Iaccheri B, Degl’ Innocenti D, Moncini D, Menchini U Invest Ophthalmol Vis Sci 49(3):1151–1156, doi:10.1167/iovs.07–0932
Wick MC, Ernestam S, Lindblad S, Bratt J, Klareskog L, van Vollenhoven RF (2005) Adalimumab (Humira) restores clinical response in patients with secondary loss of efficacy from infliximab (Remicade) or etanercept (Enbrel): results from the STURE registry at Karolinska University Hospital. Scand J Rheumatol 34(5):353–358, doi:10.1080/03009740510026887
Bennett AN, Peterson P, Zain A et al (2005) Adalimumab in clinical practice. Outcome in 70 rheumatoid arthritis patients, including comparison of patients with and without previous anti-TNF exposure. Rheumatology 44(8):1026–1031, doi:10.1093/rheumatology/keh673
Kazi AA, Jermak CM, Peyman GA et al (2006) Intravitreal toxicity of levofloxacin and gatifloxacin. Ophthalmic Surg Lasers Imaging 37(3):224–229
Manzano RP, Peyman GA, Khan P et al (2006) Testing intravitreal toxicity of bevacizumab (Avastin). Retina 26(3):257–261, doi:10.1097/00006982–200603000–00001
Yu SY, Damico FM, Viola F et al (2006) Retinal toxicity of intravitreal triamcinolone acetonide: a morphological study. Retina 26(5):531–536, doi:10.1097/00006982–200605000–00006
Kim SJ, Adams NA, Toma HS et al (2008) Safety of intravitreal ketorolac and diclofenac: an electroretinographic and histopathologic study. Retina 28(4):595–605, doi:10.1097/IAE.0b013e31815e98a5
Giansanti F, Ramazzotti M, Vannozzi L et al (2008) A pilot study on ocular safety of intravitreal infliximab in a rabbit model. Invest Ophthalmol Vis Sci 49(3):1151–1156, doi:10.1167/iovs.07–0932
Lang Y, Zemel E, Miller B et al (2007) Retinal toxicity of intravitreal kenalog in albino rabbits. Retina 27(6):778–788, doi:10.1097/IAE.0b013e318030c517
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsilimbaris, M., Diakonis, V.F., Naoumidi, I. et al. Evaluation of potential retinal toxicity of adalimumab (Humira). Graefes Arch Clin Exp Ophthalmol 247, 1119–1125 (2009). https://doi.org/10.1007/s00417-009-1065-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-009-1065-y