Abstract
Background
Diabetes mellitus is a heterogeneous metabolic disorder characterized by hyperglycemia. It is often associated with complications, such as cataracts. Cataract, characterized by cloudiness or opacity of the eye lens, is the leading cause of blindness worldwide.
Methods
The present study investigated the effect of lutein, alone or combined with insulin on the progression of eye lens opacities in streptozotocin-diabetic rats for a period of 12 weeks. Tissue markers of oxidative stress were also determined at the end of the experiment.
Results
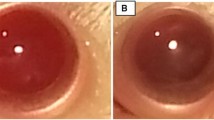
Herein we demonstrate that lutein treatment prevents the development and progression of cataracts (0 eyes with mature cataract, and ten out of 16 eyes with clear lenses in the lutein-treated diabetic animals group, vs. seven and three eyes in the non-treated diabetic group, respectively). Lipid peroxidation is significantly increased in diabetic lens (up to three-fold); lutein and insulin, alone or in combination, are able to prevent this alteration. Only insulin and lutein together could prevent the diabetes-induced decrease of glutathione content.
Conclusions
The combined treatment with lutein and insulin is useful in preventing the development of cataracts in streptozotocin-induced diabetic rats, supporting its utility in diabetes management, especially when a tight metabolic control is difficult to achieve.




Similar content being viewed by others
References
Giugliano D, Ceriello A, Paoliso G (1995) Diabetes mellitus, hypertension and cardiovascular disease. Which role for oxidative stress? Metabolism 44:363–368 doi:10.1016/0026-0495(95)90167-1
Sies H (1985) Oxidative stress: introductory remarks. In: Sies H (ed) Oxidative stress. Academic Press, London, pp 1–8
Altomare E, Vendemiale G, Grattagliano I, Angelini P, Micelli-Ferrari T, Cardia L (1995) Human diabetic cataract: role of lipid peroxidation. Diabetes Metab 21:173–179
Altomare E, Grattagliano I, Vendemiale G, Micelli-Ferrari T, Signorile A, Cardia L (1997) Oxidative protein damage in human diabetic eye: evidence of a retinal participation. Eur J Clin Invest 27:141–147 doi:10.1046/j.1365-2362.1997.780629.x
Boscia F, Grattagliano I, Vendemiale G, Micelli-Ferrari T, Altomare E (2000) Protein oxidation and lens opacity in humans. Invest Ophthalmol Vis Sci 41:2461–2465
Bhuyan KC, Bhuyan DK (1984) Molecular mechanism of cataractogenesis 3. Toxic metabolites of oxygen as initiators of lipid peroxidation and cataracts. Current Eye Res 3:67–81
Kubo E, Miyoshi N, Fukuda M, Akagi Y (1999) Cataract formation through the polyol pathway is associated with free radical production. Exp Eye Res 68:457–464 doi:10.1006/exer.1998.0624
Kyselova Z, Gajdosik A, Gajdosikova A, Ulicna O, Mijalova D, Karasu C et al (2005) Effect of the piridoindole antioxidant estobadine on development of experimental cataract and on lens protein oxidation in rats: comparison with vitamin E and BHT. Mol Vis 11:56–65
Miranda M, Muriach M, Romá J, Bosch-Morell F, Genovés JM, Barcia J et al (2006) Oxidative stress in a model of experimental diabetic retinopathy. II. Peroxynitrite scavengers utility. Arch Soc Esp Oftalmol 80:27–32
Muriach M, Bosch-Morell F, Alexander G, Blomhoff R, Barcia J, Arnal E et al (2006) Lutein effect on retina and hippocampus of diabetic mice. Free Radic Biol Med 41:979–984 doi:10.1016/j.freeradbiomed.2006.06.023
Suryanarayana P, Krishnaswamy K, Reddy GB (2003) Effect of curcumin on galactose-induced cataractogenesis in rats. Mol Vis 9:223–230
Richard MJ, Guiraud P, Meo J, Favier A (1992) High-performance liquid chromatography separation of malondialdehyde thiobarbituric acid adduct in biological materials (plasma and human cell) using a commercially available reagent. J Chromatogr A 577:9–18 doi:10.1016/0378-4347(92)80593-F
Romero MJ, Bosch-Morell F, Romero B, Rodrigo JM, Serra MA, Romero F (1998) Serum malondialdehyde: possible use for the clinical management of chronic hepatitis C patients. Free Radic Biol Med 25:993–997 doi:10.1016/S0891-5849(98)00118-X
Lawrence RA, Parkhill LK, Burk RF (1978) Hepatic cytosolic non-selenium dependent glutathione peroxidase activity: its nature and the effect of selenium deficiency. J Nutr 108:981–987
Reed DJ, Babson JR, Beatty PW, Brodie AE, Ellis WW, Potter DW (1980) High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related disulfides. Anal Biochem 106:55–62 doi:10.1016/0003-2697(80)90118-9
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lyle BJ, Mares-Perlman JA, Klein BE, Klein R, Greger JL (1999) Antioxidant intake and risk of incident age-related nuclear cataracts in the Beaver Dam Eye Study. Am J Epidemiol 149:801–809
Xing KY, Low MF (2002) Effect of H2O2 on human lens epithelial cells and the possible mechanism for oxidative damage repair by thiol transferase. Exp Eye Res 74:113–122 doi:10.1006/exer.2001.1103
Spector A, Garner WH (1981) Hydrogen peroxide and human cataract. Exp Eye Res 33:673–681 doi:10.1016/S0014-4835(81)80107-8
Cheng HM, Gonzalez RG (1986) The effect of high glucose and oxidative stress on lens metabolism, aldose reductase, and senile cataractogenesis. Metabolism 35:10–14 doi:10.1016/0026-0495(86)90180-0
Ahmed FN, Naqvi FN, Shafiq F (2006) Lipid peroxidation and serum antioxidant enzymes in patients with type II diabetes mellitus. Ann N Y Acad Sci 1084:481–489 doi:10.1196/annals.1372.022
Riley ML, Harding JJ (1993) The reaction of malondialdehyde with lens proteins and the protective effect of aspirin. Biochim Biophys Acta 1158:107–112
Libondi T, Ragone R, Vicenti D, Stiuso P, Auricchio G, Colonna G (1994) In vitro crosslinking of calf lens alpha-crystallin by malondialdehyde. Int J Pept Protein Res 44:342–347
Suryanarayana P, Saraswat M, Petrash JM, Reddy JB (2007) Emblica officinalis and its enriched tanoids delay streptozotocin-induced diabetic cataract in rats. Mol Vis 13:1291–1297
Cekic O, Bardak Y, Totan Y, Akyol O, Zilelioglu G (1999) Superoxide dismutase, catalase, glutathione peroxidase and xanthin oxidase in diabetic rat lenses. Ophthalmic Res 31:346–350 doi:10.1159/000055557
Khana P, Wang L, Perez-Polo RJ, Ansari NH (1997) Oxidative defense, enzyme activity and mRNA levels in lenses of diabetic rats. J Toxicol Environ Health 51:6–10
Calvin HI, Medvedovsky C, Worgul BV (1986) Near-total glutathione depletion and age specific cataract induced by buthionine sulphoximine in mice. Science 233:553–555 doi:10.1126/science.3726547
Micelli-Ferrari T, Vendemiale G, Grattagliano I, Boscia P, Arnese S, Altomare E et al (1996) Role of lipid peroxidation in the pathogenesis of myopic and senile cataract. Br J Ophthalmol 80:840–843 doi:10.1136/bjo.80.9.840
Acknowledgements
This study was partially supported with funds from Fundación San Pablo-CEU. Thanks are also indebted to Kemin Health L.C. for its support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arnal, E., Miranda, M., Almansa, I. et al. Lutein prevents cataract development and progression in diabetic rats. Graefes Arch Clin Exp Ophthalmol 247, 115–120 (2009). https://doi.org/10.1007/s00417-008-0935-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-008-0935-z