Abstract
Purpose
Abnormal angiogenesis is the hallmark feature of retinopathy of prematurity (ROP), and contributes to the severe visual loss that accompanies this disease. Thalidomide is a well-known anti-angiogenic drug. We tested the assumption that injection of intraperitoneal thalidomide could reduce the severity of oxygen-induced retinopathy (OIR) in a mouse model.
Methods
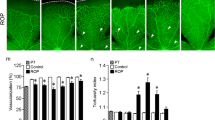
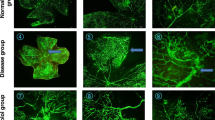
Forty-three baby wild type mice were used in this study. The mouse model of oxygen-induced retinopathy consisted of a 5-day exposure to 75% oxygen from postnatal day 7 to 12 (P12) followed by 5 days in room air (relative hypoxia). Control mice were those with normally developing retinal vasculature exposed to room air from birth until postnatal day 17 (P17). Thalidomide (200 mg/Kg) was administered daily intraperitoneally to control and ROP mice in two protocols: (1) from P12 to P16, and (2) from P11 to P15 . Fluorescein-conjugated dextran angiography of retinal vasculature was performed on P17, and retinal whole mounts were prepared to score features of retinopathy. The parameters that were scored in a masked fashion included blood vessel growth, blood vessel tufts formation, extra retinal neovascularization, degree of central constriction, and tortuosity of vessels. These parameters constitute the Modified Retinopathy Scoring System (MRSS). In addition, quantification of the number of blood vessel tufts was performed in a masked fashion with hematoxylin & eosin (H&S) staining of paraffin-embedded eye sections.
Results
The retinopathy score by MRSS in the thalidomide treated mice was similar to that of untreated mice that were exposed to oxygen (9.3 ± 1.9 vs 10.15 ± 1.6; p = 0.21). The neovascularization count was also similar between the two groups (10.4 ± 5.6 vs 9.6 ± 4.8; p = 0.56). In the control group left in the room air, the retinopathy score was 0.19 ± 0.37 (p = 0) and the neovascularization count was also very low (2.92 ± 2.14; p = 0).
Conclusions
Although thalidomide might have a proven anti-angiogenic and anti-inflammatory effect, our model did not show a significant effect on the retinopathy. The reason might be an ineffective level of the drug in the retina due to ineffective metabolism of the drug, or due to blockage of the drug by the blood-retina barrier, or the involvement of other factors besides those influenced by thalidomide in the process.







Similar content being viewed by others
References
Aydogan S, Celiker U, Turkcuoglu P, Ilhan N, Akpolat N (2007) The effect of thalidomide on vascular endothelial growth factor and tumor necrosis factor-alpha levels in retinal ischemia/reperfusion injury. Graefes Arch Clin Exp Ophthalmol. Sep 3 (Epub ahead of print)
Calabrese L, Fleicher AB (2000) Thalidomide: current and potential clinical applications. Am J Med 108(6):487–495
Carlesimo M, Giustini S, Rossi A, Bonaccorsi P, Calvieri S (1995) Treatment of cutaneous and pulmonary sarcoidosis with thalidomide. J Am Acad Derm 32:866–869
Cryotheraphy for Retinopathy of Prematurity Cooperative Group (1996) Multicenter trial of cryotheraphy for retinopathy of prematurity. Snellen visual acuity and structural outcome at 5½ after randomization. Arch Ophthalmol 114:417–424
Cryotheraphy for Retinopathy of Prematurity Cooperative Group (2001) Multicenter trial of cryotheraphy for retinopathy of prematurity. Ophthalmologic outcomes at 10 years. Arch Ophthalmol 119:1110–1118
Curtis EM, Amy L (1995) Fixation of whole eyes: the role of fixative osmolarity in the production of tissue artifact. Graefes Arch Clin Exp Ophthalmol 233:366–370
D’Amato R, Weselowsk E, Smith LEH (1993) Microscopic visualization of the retina by angiography with high molecular weight fluorescein labeled dextrans in the mouse. Microvas Res 46:135–142
D’Amato RJ, Loughnan MS, Flynn E, Folkman J (1994) Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA 91:4082–4085
Gelati M, Corsini E, Frigerio S, Pollo B, Broggi G, Croci D (2003) Effects of thalidomide on parameters involved in angiogenesis: an in vitro study. J Neurooncol 64(3):193–201
Gradner-Medwin JM, Smith NJ, Powell RJ (1994) Clinical experience with thalidomide in the management of severe oral and genital ulceration in conditions such as Behcet’s disease: use of neurophysiological studies to detect thalidomide neuropathy. Ann Rheumatol Disease 53(12):828–832
Guex-Crosier Y, Pittet N, Herbort CP (1995) The effect of Thalidomide and Sulpidimide on endotoxin-induced uveitis in rats. Graefes Arch Clin Exp Ophthalmol 233(2):990–993
Higgins RD et al (1999) Diltiasem reduces retinal neovascularization in a mouse model of oxygen induced retinopathy. Curr Eye Res 18:20–27
Kaplan G, Thomas S, Fierer DS (2000) Thalidomide for the treatment of AIDS- associated wasting. AIDS Res Hum Retroviruses 16(14):1345–1355
Katz ML, Robison WJ Jr (1988) Autoxidative damage to the retina: potential role in retinopathy of prematurity. Birth Defects Orig Artic Ser 24:237–248
Kenyon BM, Brown F, D’Amato RJ (1997) Effects of thalidomide and related metabolites in a mouse corneal model of neovascularization. Exp Eye Res 64:971–978
Kohler F, Ockenfels H (1970) Teratogene Wirkung der N-Phthalyl-DL-glutamisaure nach intraperitonealern Applikation bei der Maus. Experientia 26:1157–1158
Lenz W (1962) Thalidomide and congenital abnormalities. Lancet 1:45
McBride WG (1961) Thalidomide and congenital abnormalities. Lancet 2:1358
McCarthy MF (1997) Thalidomide may impede cell migration in primates by down regulating integrin beta-chains: potential therapeutic utility in solid malignancies, proliferative retinopathy, inflammatory disorders, neointimal hyperplasia, and osteoporosis. Med Hypotheses 49:123–131
Penn JS, Rajaratnam VS, Collier RJ, Clark AF (2001) The effect of an angiostatic steroid on neovascularization in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci 42(1):283–290
Phelps DL (1992) Retinopathy of prematurity. Curr Probl Pediatr 22:349–371
Pierce EA, Foley ED, Smith LE (1996). Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol 114(10):1219–1228
Sauer H, Gunther J, Hescheler J, Wartenberg M (2000) Thalidomide inhibits angiogenesis in embryoid bodies by the generation of hydroxyl radicals. Am J Pathology 156:151–158
Scott WJ, Fradkin R, Wilson JG (1970) Nonconfirmation of thalidomide induced teratogenesis in rats and mice. Teratology 5:33–36
Sheskin J (1980) The treatment of lepra reactions in lepromatous leprosy. Fifteen years’ experience with thalidomide. Int J Der 19:318–322
Smith LEH (1994) Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 35:101–111
Spierer A, Rabinowitz R, Pri-Chen S, Rosner M (2005) An increase in superoxide dismutase ameliorates oxygen-induced retinopathy in transgenic mice. Eye 19:86–91
Stone J, Chan-Ling T, Pe’er J (1996) Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Invest Ophthalmol Vis Sci 37:290–299
Szabo KT, Steelman RL (1967) Effects of maternal thalidomide treatment on pregnancy, fetal development, and mortality of the offsprings in random-bred mice. Am J Vet Res 28(127):1823–1828
Tseng S, Pak G, Washenik K (1996) Rediscovering thalidomide: A review of its mechanism of action, side effects, and potential uses. J Am Acad Dermatol 35(6):969–979
Vogelsang GB, Farmer ER, Hess AD, Altamonte V, Beschoner WE, Jabs DA (1992) Thalidomide for the treatment of chronic graft-versus-host disease. N Engl J Med 326:1055–1058
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rabinowitz, R., Katz, G., Rosner, M. et al. The effect of thalidomide on neovascularization in a mouse model of retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 246, 843–848 (2008). https://doi.org/10.1007/s00417-008-0781-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-008-0781-z