Abstract
Purpose
To examine the effects of focal laser photocoagulation on general and local retinal function and to relate electrophysiological findings with changes in protein kinase C (PKC) alpha expression.
Methods
Twelve rabbits were treated with 70 spots of laser photocoagulation in the central cone-rich retina. The operated eyes were investigated with electroretinography (full-field ERG and multifocal electroretinography, mfERG) preoperatively and at 1, 3, and 5 weeks after surgery. The expression of PKC alpha was examined at all three time points using immunohistochemistry, and PKC alpha mRNA levels were quantified using real-time polymerase chain reaction (PCR). Immunohistochemistry for glial fibrillary acidic protein (GFAP) and hematoxylin and eosin staining was employed to monitor the extent and dynamics of the morphological response.
Results
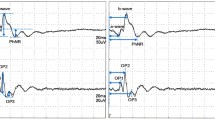
The full-field ERG revealed a significant increase in b-wave amplitudes derived from the isolated rod response (blue light) at all three time points after surgery (p < 0.05). Supernormal b-wave amplitudes were also found for the combined rod–cone response at 3 weeks (white light), and for the isolated cone response (light-adapted 30-Hz flicker) at 5 weeks after treatment. In the mfERG, amplitudes derived from the central retina did not change postoperatively, while the implicit time was significantly increased at all time points. Immunohistochemistry for PKC alpha revealed a reduced expression of the enzyme in rod bipolar cells 1 and 3 weeks after laser treatment compared with untreated controls. Five weeks postoperatively, no PKC alpha labeling in rod bipolar cells was found in any part of the retina. Real-time PCR 1 and 3 weeks after treatment displayed a decreased level of PKC alpha mRNA compared to the controls. Immunolabeled tissue sections from laser-treated eyes displayed GFAP expression in Müller cells in the treated as well as untreated retina 1 week postoperatively. At 3 and 5 weeks, GFAP labeling was less pronounced and was concentrated around the laser-treated spots.
Conclusions
Focal laser treatment in the rabbit eye induces local and wide-spread alterations in both rod- and cone-mediated retinal function in the form of supernormal b-wave amplitudes in the full-field ERG and increased latency in the mfERG. The electrophysiological abnormalities are accompanied by a progressive down-regulation of the PKC alpha isoenzyme in rod bipolar cells, reaching far beyond the treated area. PKC alpha is down-regulated directly by impaired protein synthesis, and also possibly indirectly by protein consumption related to GFAP up-regulation. The results indicate that focal laser photocoagulation interferes with PKC-alpha-mediated inhibitory regulation of inner retinal signal transmission.






Similar content being viewed by others
References
Bettler B, Mulle C (1995) Review: neurotransmitter receptors. II. AMPA and kainate receptors. Neuropharmacology 34:123–139
Brodie C, Kuperstein I, Acs P, Blumberg PM (1998) Differential role of specific PKC isoforms in the proliferation of glial cells and the expression of the astrocytic markers GFAP and glutamine synthetase. Brain Res Mol Brain Res 56:108–117
Chuang HC, Kawano S, Arai M, Tsukada T, Kita M, Negi A, Honda Y (1992) The influence of argon laser panretinal photocoagulation on the rabbit ERG c-wave. Acta Ophthalmol (Copenh) 70:303–307
Ciavarella P, Moretti G, Falsini B, Porciatti V (1997) The pattern electroretinogram (PERG) after laser treatment of the peripheral or central retina. Curr Eye Res 16:111–115
Early Treatment Diabetic Retinopathy Study Research Group (1985) Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol 103:1796–1806
Ehinger B, Falck B (1971) Autoradiography of some suspected neurotransmitter substances: GABA glycine, glutamic acid, histamine, dopamine, and L-dopa. Brain Res 33:157–172
Famiglietti EV, Sharpe SJ (1995) Regional topography of rod and immunocytochemically characterized “blue” and “green” cone photoreceptors in rabbit retina. Vis Neurosci 12:1151–1175
Gerth C, Hauser D, Delahunt PB, Morse LS, Werner JS (2003) Assessment of multifocal electroretinogram abnormalities and their relation to morphologic characteristics in patients with large drusen. Arch Ophthalmol 121:1404–1414
Ghosh F, Gjörloff K (2005) Protein kinase C expression in the rabbit retina after laser photocoagulation. Graefes Arch Clin Exp Ophtahalmol 243:803–810
Gjörloff K, Andréasson S, Ghosh F (2006) mfERG in normal and lesioned rabbit retina. Graefes Arch Clin Exp Ophtahalmol 244:83–89
Gjörloff KW, Andréasson S, Ehinger B (2004) Standardized full-field electroretinography in rabbits. Doc Opthalmol 109:163–168
Gottlob I, Wündsch L, Tuppy FK (1988) The rabbit electroretinogram: effect of GABA and its antagonists. Vision Res 28:203–210
Greenstein VC, Chen H, Hood DC, Holopigian K, Seiple W, Carr RE (2000) Retinal function in diabetic macular edema after focal laser photocoagulation. Invest Ophthalmol Vis Sci 41:3655–3664
Hammer RM, Yinon U, Rosner M, Solomon A (1996–1997) Time course of electroretinographic recovery following argon laser photocoagulation in cats. Metab Pediatr Syst Ophthalmol 19–20:1–5
Henricsson M, Heijl A (1994) The effect of panretinal laser photocoagulation on visual acuity, visual fields and on subjective visual impairment in preproliferative and early proliferative diabetic retinopathy. Acta Ophthalmol (Copenh) 72:570–575
Hood DC, Cideciyan AV, Halevy DA, Jacobson SG (1996) Sites of disease action in a retinal dystrophy with supernormal and delayed rod electroretinogram b-waves. Vision Res 36:889–901
Humphrey MF, Constable IJ, Chu Y, Wiffen S (1993) A quantitative study of the lateral spread of Müller cell responses to retinal lesions in the rabbit. J Comp Neurol 334:545–558
Ikeda H, Franchi A, Turner G, Shilling J, Graham E (1989) Electroretinography and electro-oculography to localize abnormalities in early-stage inflammatory eye disease. Doc Ophthalmol 73:387–394
Johansson K, Malmsjö M, Ghosh F (2006) Tailored vitrectomy and laser photocoagulation without scleral buckling for all primary rhegmatogenous retinal detachments. Br J Ophtalmol 90:1286–1291
Jung C-S, Lee S-J, Paik S-S, Bai S-H (2000) Run-up of gamma-aminobutyric acid(C) responses in catfish retinal cone-horizontal cell axon-terminals is modulated by protein kinase A and C. Neurosci Lett 282:53–56
Khosla PK, Rao V, Tewari HK, Kumar A (1994) Contrast sensitivity in diabetic retinopathy after panretinal photocoagulation. Ophthalmic Surg 25:516–520
Leibu R, Davila E, Zemel E, Bitterman N, Miller B, Perlman I (1999) Development of laser-induced retinal damage in the rabbit. Graefes Arch Clin Exp Ophtahalmol 237:991–1000
Lewis GP, Fisher SK (2003) Up-regulation of glial fibrillary acidic protein in response to retinal injury: its potential role in glial remodeling and a comparison to vimentin expression. Int Rev Cytol 230:263–290
Marshall J (1989) Structural aspects of laser-induced damage and their functional implications. Health Phys 56:617–624
Michaelides M, Holder GE, Webster AR, Hunt DM, Bird AC, Fitzke FW, Mollon JD, Moore AT (2005) A detailed phenotypic study of “cone dystrophy with supernormal rod ERG”. Br J Ophtalmol 89:332–339
Nicol GD, Miller WH (1978) Cyclic GMP injected into retinal rod outer segments increases latency and amplitude of response to illumination. Proc Natl Acad Sci USA 75:5217–5220
Noetzel MJ (1990) Phosphorylation of the glial fibrillary acidic protein. J Neurosci Res 27:184–192
Osborne NN, Barnett NL (1992) An intraocular injection of kainate induces expression of c-fos-like protein and activation of protein kinase C (alpha) in specific rabbit retinal neurones. Brain Res Mol Brain Res 15:108–112
Osborne NN, Broyden NJ, Barnett NL, Morris NJ (1991) Protein kinase C (alpha and beta) immunoreactivity in rabbit and rat retina: effect of phorbol esters and transmitter agonists on immunoreactivity and the translocation of the enzyme from cytosolic to membrane compartments. Neurochem 57:594–604
Osborne NN, Wood J, Muller A (1995) The influence of experimental ischaemia on protein kinase C and the GABAergic system in the rabbit retina. Neuropharmacology 34:1279–1288
Pawlyk BS, Sandberg MA, Berson EL (1991) Effects of IBMX on the rod ERG of the isolated perfused cat eye: antagonism with light, calcium or L-cis-diltiazem. Vision Res 31:1093–1097
Pender PM, Benson WE, Compton H, Cox GB (1981) The effects of panretinal photocoagulation on dark adaptation in diabetics with proliferative retinopathy. Ophthalmology 88:635–638
Rosner M, Solberg Y, Turetz J, Belkin M (1997) Neuroprotective therapy for argon-laser induced retinal injury. Exp Eye Res 65:485–495
Saran BR, Brucker AJ (1995) Macular epiretinal membrane formation and treated retinal breaks. Am J Ophthalmol 120:480–485
Schechner R, Gdal-on M, Cohen D, Meyer E, Zonis S, Perlman I (1987) Recovery of the electroretinogram in rabbits after argon laser photocoagulation. Invest Ophthalmol Vis Sci 28:1605–1613
Stanford MR, Robbins J, Kasp E, Dumonde DC (1992) Passive administration of antibody against retinal S-antigen induces electroretinographic supernormality. Invest Ophthalmol Vis Sci 33:30–35
The Branch Vein Occlusion Study Group (1984) Argon laser photocoagulation for macular edema in branch vein occlusion. Am J Ophthalmol 98:271–282
The Macular Photocoagulation Study Group (1990) Krypton laser photocoagulation for neovascular lesions of age-related macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol 108:816–824
Tyrberg M, Ponjavic V, Lövestam-Adrian M (2005) Multifocal electroretinography (mfERG) in insulin dependent diabetics with and without clinically apparent retinopathy. Doc Ophthalmol 110:137–143
Vaquero CF, Velasco A, de la Villa P (1996) Protein kinase C localization in the synaptic terminal of rod bipolar cells. Neuroreport 7:2176–2180
Virgili M, Contestabile A, Barnabei O (1995) Simultaneous blockade of non-NMDA ionotropic receptors and NMDA receptor-associated ionophore partially protects hippocampal slices from protein synthesis impairment due to simulated ischemia. Hippocampus 5:91–97
Wolbarsht ML, Landers MB 3rd (1980) The rationale of photocoagulation therapy for proliferative diabetic retinopathy: a review and a model. Ophthalmic Surg 11:235–245
Wood JPM, McCord RJ, Osborne NN (1997) Retinal protein kinase C. Neurochem Int 30:119–136
Acknowledgments
The authors would like to extend their gratitude towards Boel Nilsson and Karin Arnér for their skilled technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Faculty of Medicine, Lund University, Crown Princess Margareta’s Foundation for the Visually Impaired, the Swedish Research Council, Maggie Stephens Foundation, Synfrämjandets Forskningsfond, the Åke Wiberg Foundation, the M. Bergvall Foundation, Anna Lisa and Sven-Eric Lundgrens Foundation for Medical Research, the Anders Otto Swärds Foundation, Ulrika Eklunds Foundation, and the Swedish Medical Association. The authors have no financial relationship with the sponsoring organizations.
The authors have full control of all primary data and agree to allow Graefe’s Archive for Clinical and Experimental Ophthalmology to review the data if requested.
Rights and permissions
About this article
Cite this article
Wallentén, K.G., Malmsjö, M., Andréasson, S. et al. Retinal function and PKC alpha expression after focal laser photocoagulation. Graefes Arch Clin Exp Ophthalmol 245, 1815–1824 (2007). https://doi.org/10.1007/s00417-007-0646-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-007-0646-x