Abstract
Background
Diabetes inhibits dark adaptation and both processes alter the electroretinogram (ERG) in similar ways. This study aimed to investigate the relationship between oscillatory potentials (OPs) and the b-wave during dark adaptation and to determine if this relationship changes during the development of diabetes.
Methods
Twenty-one rats were assigned to adaptation, control and diabetic groups. Rats were dark adapted for periods between 20 minutes and 4 hours, and ERGs recorded. Diabetes was induced with streptozotocin, and ERGs measured after 3, 6, 9 and 12 weeks after injection.
Results
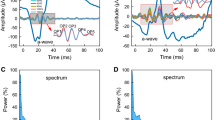
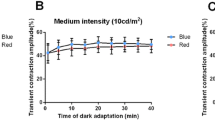
Increasing periods of dark adaptation led to a logarithmic increase in the amplitude of the b-wave and the OPs. This was accompanied by a decrease in the peak times of the OPs and b-wave. Total OP amplitude and b-wave amplitude were linearly related, allowing an empirical OP constant to be developed to describe the relationship between the two parameters. Diabetes led to a progressive decrease in the amplitude and increase in the peak time of all waves. The OP constant decreased in a linear fashion with increasing duration of diabetes.
Conclusions
It is argued that OP masking of the b-wave could explain previous inconsistencies in reported ERG changes in diabetes and that a slowing of dark adaptation does not account for these ERG changes. The report concludes that the OPs and b-wave amplitudes and latencies are intimately related in the normal retina and that this correlation is lost predictably during the development of diabetes.





Similar content being viewed by others
References
Aizu Y, Oyanagi K, Hu J, Nakagawa H (2002) Degeneration of retinal neuronal processes and pigment epithelium in the early stage of the streptozotocin-diabetic rats. Neuropathology 22:161–170
Amemiya T (1977) Dark adaptation in diabetics. Ophthalmologica 174:322–326
Arden GB, Wolf JE, Tsang Y (1998) Does dark adaptation exacerbate diabetic retinopathy? Evidence and a linking hypothesis. Vis Res 38:1723–1729
Asi H, Perlman I (1992) Relationships between the electroretinogram a-wave, b-wave and oscillatory potentials and their application to clinical diagnosis. Doc Ophthalmol 79:125–139
Benoit J, Lachapelle P (1990) Temporal relationship between ERG components and geniculate unit activity in rabbit. Vis Res 30:797–806
Biro K, Palhalmi J, Toth AJ, Kukorelli T, Juhasz G (1998) Bimoclomol improves early electrophysiological signs of retinopathy in diabetic rats. Neuroreport 9:2029–2033
Brooks DE, Sims MH, Gum GG, Blackstock MJ (1992) Changes in oscillatory potentials of the canine electroretinogram during acute sequential elevations in intraocular pressure. Prog Vet Comp Ophthalmol 2:80–89
Brunette JR, Lafond G (1983) Effects of dark adaptation on implicit time in the clinical electroretinogram. Can J Ophthalmol 18:33–36
Bui BV, Armitage JA, Tolcos M, Cooper ME, Vingrys AJ (2003) ACE inhibition salvages the visual loss caused by diabetes. Diabetologia 46:401–408
Dick E, Miller RF (1978) Light-evoked potassium activity in mudpuppy retina: its relationship to the b-wave of the electroretinogram. Brain Res 154:388–394
Feghali JG, Jin JC, Odom JV (1991) Effect of short-term intraocular pressure elevation on the rabbit electroretinogram. Invest Ophthalmol Vis Sci 32:2184–2189
Granit R (1933) Components of the retinal action potentials in mammals and their relation to the discharge in the optic nerve. J Physiol 77:207–239
Hancock HA, Kraft TW (2004) Oscillatory potential analysis and ERGs of normal and diabetic rats. Invest Ophthalmol Vis Sci 45:1002–1008
Henson DB, North RV (1979) Dark adaptation in diabetes mellitus. Br J Ophthalmol 63:539–541
Hotta N, Koh N, Sakakibara F, Nakamura J, Hara T, Hamada Y, Fukasawa H, Kakuta H, Sakamoto N (1997) Effect of an aldose reductase inhibitor on abnormalities of electroretinogram and vascular factors in diabetic rats. Eur J Pharmacol 326:45–51
Hotta N, Koh N, Sakakibara F, Nakamura J, Hamara Y, Hara T, Nakashima E, Sasaki H, Fukasawa H, Kakuta H, Sakamoto N (1996) Effects of propionyl-L-carnitine and insulin on the electroretinogram, nerve conduction and nerve blood flow in rats with streptozotocin-induced diabetes. Pflugers Arch 431:564–570
Hotta N, Koh N, Sakakibara F, Nakamura J, Hamada Y, Hara T, Mori K, Nakashima E, Naruse K, Fukasawa H, Kakuta H, Sakamoto N (1996) Effects of beraprost sodium and insulin on the electroretinogram, nerve conduction, and nerve blood flow in rats with streptozotocin-induced diabetes. Diabetes 45:361–366
Keller C, Grimm C, Wenzel A, Hafezi F, Reme C (2001) Protective effect of halothane anesthesia on retinal light damage: inhibition of metabolic rhodopsin regeneration. Invest Ophthalmol Vis Sci 42:476–480
Lachapelle P (1987) Analysis of the photopic electroretinogram recorded before and after dark adaptation. Can J Ophthalmol 22:354–361
Lachapelle P (1990) Oscillatory potentials as predictors to amplitude and peak time of the photopic b-wave of the human electroretinogram. Doc Ophthalmol 75:73–82
Lachapelle P (1991) Evidence for an intensity-coding oscillatory potential in the human electroretinogram. Vis Res 31:767–774
Marmor MF, Zrenner E (1998) Standard for clinical electroretinography (1999 update). International Society for Clinical Electrophysiology of Vision. Doc Ophthalmol 97:143–156
Marmor MF, Holder GE, Seeliger MW, Yamamoto S (2004) Standard for clinical electroretinography (2004 update). Doc Ophthalmol 108:107–114
Miller RF, Dowling JE (1970) Intracellular responses of the Muller (glial) cells of mudpuppy retina: their relation to b-wave of the electroretinogram. J Neurophysiol 33:323–341
Miyake Y, Solish A, Hara A, Hirose T (1978) Clinical application of a digital computer software system to electrodiagnosis. Ophthalmic Res 10:268–278
Noell WK, Delmelle MC, Albrecht R (1971) Vitamin A deficiency effect on retina: dependence on light. Science 172:72–75
Ogden TE (1973) The oscillatory waves of the primate electroretinogram. Vis Res 13:1059–1074
Ostroy SE (1998) Altered rhodopsin regeneration in diabetic mice caused by acid conditions within the rod photoreceptors. Curr Eye Res 17:979–985
Ostroy SE, Frede SM, Wagner EF, Gaitatzes CG, Janle EM (1994) Decreased rhodopsin regeneration in diabetic mouse eyes. Invest Ophthalmol Vis Sci 35:3905–3909
Perlman I (1983) Relationship between the amplitudes of the b-wave and the a wave as a useful index for evaluating the electroretinogram. Br J Ophthalmol 67:443–448
Rapp LM, Basinger SF (1982) The effects of local anaesthetics on retinal function. Vis Res 22:1097–1103
Sakai H, Tani Y, Shirasawa E, Shirao Y, Kawasaki K (1995) Development of electroretinographic alterations in streptozotocin-induced diabetes in rats. Ophthalmic Res 27:57–63
Seki M, Tanaka T, Nawa H, Usui T, Fukuchi T, Ikeda K, Abe H, Takei N (2004) Involvement of brain-derived neurotrophic factor in early retinal neuropathy of streptozotocin-induced diabetes in rats: therapeutic potential of brain-derived neurotrophic factor for dopaminergic amacrine cells. Diabetes 53:2412–2419
Sims MH (1990) Partial masking of the canine electroretinogram by oscillatory potentials: The problem of frequency bandwidth. J Vet Intern Med 4:40–42
Sims MH, Brooks DE (1990) Changes in oscillatory potentials of the canine electroretinogram during dark adaptation. Am J Vet Res 51:1580–1586
Stodmeister R (1973) The spectral sensitivity functions of human ERG wavelets. Ophthalmic Res 5:21–30
Strackee J, Van Der Tweel L (1974) Data analysis of electrophysiological signals. Doc Ophthalmol, Proc Ser 10:23–35
Tuitoek PJ, Ziari S, Tsin AT, Rajotte RV, Suh M, Basu TK (1996) Streptozotocin-induced diabetes in rats is associated with impaired metabolic availability of vitamin A (retinol). Br J Nutr 75:615–622
Tzekov R, Arden GB (1999) The electroretinogram in diabetic retinopathy. Surv Ophthalmol 44:53–60
Vadala M, Anastasi M, Lodato G, Cillino S (2002) Electroretinographic oscillatory potentials in insulin-dependent diabetes patients: A long-term follow-up. Acta Ophthalmol Scand 80:305–309
Van Der Torren K, Groeneweg G, Van Lith G (1988) Measuring oscillatory potential during the course of an arterial occlusion: a method based on Fourier analysis. Doc Ophthalmol 70:199–203
Wachtmeister L (1998) Oscillatory potentials in the retina: what do they reveal. Prog Retin Eye Res 17:485–521
Wachtmeister L, Dowling JE (1978) The oscillatory potentials of the mudpuppy retina. Invest Ophthalmol Vis Sci 17:1176–1188
Xu X, Karwoski CJ (1994) Current source density (CSD) analysis of retinal field potentials. I. Methodological considerations and depth profiles. J Neurophysiol 72:84–95
Xu X, Karwoski CJ (1994) Current source density analysis of retinal field potentials. II. Pharmacological analysis of the b-wave and M-wave. J Neurophysiol 72:96–105
Acknowledgements
Nigel Switelski for his laboratory assistance. CJ Layton is supported by the Rhodes Trust. The patronage of Maureen Leach, Optometrist and the Optometrists Association of Australia is appreciated. The opinions provided by Dr Robert J Casson (University of Adelaide) on the manuscript were very valuable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Layton, C.J., Safa, R. & Osborne, N.N. Oscillatory potentials and the b-Wave: Partial masking and interdependence in dark adaptation and diabetes in the rat. Graefes Arch Clin Exp Ophthalmol 245, 1335–1345 (2007). https://doi.org/10.1007/s00417-006-0506-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-006-0506-0