Abstract
Background
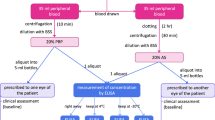
Serum eyedrops have been successfully used in the treatment of severe dry eye, persistent epithelial defects and other severe ocular surface disorders. A number of clinical studies showed a variable efficacy of this approach, but the parameters for the production of this blood product varied significantly. In order to establish an optimised protocol for the production of serum eyedrops, we examined the effect of various clotting times, centrifugation forces, types of diluent and dilutions on the concentration of growth factors, fibronectin, and vitamins in serum and tested the epitheliotrophic capacity of these serum modifications in a cell culture model of human SV-40-immortalised corneal epithelial cells (HCE-T).
Methods
Serum samples were prepared with a clotting time of 20, 60 or 120 min, a centrifugation force of 500×g or 3,000×g, and diluted with BSS or isotonic saline. The concentrations of EGF, TGF-β1, PDGF-AB, FGF, HGF, fibronectin, vitamin A and vitamin E in these samples were evaluated with ELISA and HPLC. HCE-T cells were incubated for 24, 48, 72, 96 and 144 h with 100, 50, 25, 12.5, 6.25 and 3.125% serum in diluent, and cell proliferation, migration and differentiation were evaluated by means of a luminescence-based ATP assay, a colony-dispersion assay and scanning electron microscopy.
Results
Using a longer clotting time resulted in an increased concentration of all the epitheliotrophic factors examined in serum; the diffference was statistically significant for EGF, TGF-β1 and HGF. Increasing the g force of centrifugation from 500×g to 3,000×g resulted in significantly less TGF-β1, but more EGF and vitamin A. Cell proliferation was better supported by serum prepared with 3,000×g and diluted with BSS. Serum prepared with a longer clotting time yielded better cell migration and differentiation.
Conclusion
Clotting time, centrifugation and diluents have a significant impact on the composition and epitheliotrophic effects of serum. A long clotting time (≥120 min), a sharp centrifugation (3,000×g for 15 min) and dilution with BSS improve the ability of serum eyedrops to support proliferation, migration and differentiation of corneal epithelial cells.



Similar content being viewed by others
References
Andresen JL, Ledet T, Ehlers N (1997) Keratocyte migration and peptide growth factors: the effect of PDGF, bFGF, EGF, IGF-I, aFGF and TGF-beta on human keratocyte migration in a collagen gel. Curr Eye Res 16:605–613
Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, Handa H (1995) An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci 36:614–621
Bilgihan K, Adiguzel U, Sezer C, Akyol G, Hasanreisoglu B (2001) Effects of topical vitamin E on keratocyte apoptosis after traditional photorefractive keratectomy. Ophthalmologica 215:192–196
Chandrasekher G, Kakazu AH, Bazan HE (2001) HGF- and KGF-induced activation of PI-3K/p70 s6 kinase pathway in corneal epithelial cells: its relevance in wound healing. Exp Eye Res 73:191–202
Collins MK, Perkins GR, Rodriguez-Tarduchy G, Nieto MA, Lopez-Rivas A (1994) Growth factors as survival factors: regulation of apoptosis. Bioessays 16:133–138
del Castillo JM, de la Casa JM, Sardina RC, Fernandez RM, Feijoo JG, Gomez AC, Rodero MM, Sanchez JG (2002) Treatment of recurrent corneal erosions using autologous serum. Cornea 21:781–783
Dugrillon A, Lauber S, Nguyen X, Klueter H (eds) (2002) Platelets applied to wounds and in tissue regeneration: induction of proliferation apoptosis by platelet membranes
Er H, Uzmez E (1998) Effects of transforming growth factor-beta 2, interleukin 6 and fibronectin on corneal epithelial wound healing. Eur J Ophthalmol 8:224–229
Ferreira de Souza R, Kruse FE, Seitz B (2001) Autologous serum for otherwise therapy resistant corneal epithelial defects—prospective report on the first 70 eyes. Klin Monatsbl Augenheilkd 218:720–726
Fox RI, Chan R, Mishelson JB (1984) Beneficial effect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis Rheum 27:459–461
Garlick JA, Taichman LB (1994) Fate of human keratinocytes during reepithelialization in an organotypic culture model. Lab Invest 70:916–924
Geerling G, Sieg P, Bastian GO, Laqua H (1998) Transplantation of the autologous submandibular gland for most severe cases of keratoconjunctivitis sicca. Ophthalmology 105:327–335
Geerling G, Daniels JT, Dart JK, Cree IA, Khaw PT (2001) Toxicity of natural tear substitutes in a fully defined culture model of human corneal epithelial cells. Invest Ophthalmol Vis Sci 42:948–956
Goto E, Shimmura S, Shimazaki J, Tsubota K (2001) Treatment of superior limbic keratoconjunctivitis by application of autologous serum. Cornea 20:807–810
Grant MB, Khaw PT, Schultz GS, Adams JL, Shimizu RW (1992) Effects of epidermal growth factor, fibroblast growth factor, and transforming growth factor-beta on corneal cell chemotaxis. Invest Ophthalmol Vis Sci 33:3292–3301
Haber M, Cao Z, Panjwani N, Bedenice D, Li WW, Provost PJ (2003) Effects of growth factors (EGF, PDGF-BB and TGF-beta 1) on cultured equine epithelial cells and keratocytes: implications for wound healing. Vet Ophthalmol 6:211–217
Kay EP, Lee MS, Seong GJ, Lee YG (1998) TGF-beta s stimulate cell proliferation via an autocrine production of FGF-2 in corneal stromal fibroblasts. Curr Eye Res 17:286–293
Kim JM, Stapleton F, Willcox MD (1999) Induction of apoptosis in human corneal epithelial cells in vitro. Aust N Z J Ophthalmol 27:214–217
Kim WJ, Mohan RR, Wilson SE (1999) Effect of PDGF, IL-1alpha, and BMP2/4 on corneal fibroblast chemotaxis: expression of the platelet-derived growth factor system in the cornea. Invest Ophthalmol Vis Sci 40:1364–1372
Kitazawa T, Kinoshita S, Fujita K, Araki K, Watanabe H, Ohashi Y, Manabe R (1990) The mechanism of accelerated corneal epithelial healing by human epidermal growth factor. Invest Ophthalmol Vis Sci 31:1773–1778
Li Q, Weng J, Mohan RR, Bennett GL, Schwall R, Wang ZF, Tabor K, Kim J, Hargrave S, Cuevas KH, Wilson SE (1996) Hepatocyte growth factor and hepatocyte growth factor receptor in the lacrimal gland, tears, and cornea. Invest Ophthalmol Vis Sci 37:727–739
McDonnell PJ, Schanzlin DJ, Rao NA (1988) Immunoglobulin deposition in the cornea after application of autologous serum. Arch Ophthalmol 106:1423–1425
Mishima H, Nakamura M, Murakami J, Nishida T, Otori T (1992) Transforming growth factor-beta modulates effects of epidermal growth factor on corneal epithelial cells. Curr Eye Res 11:691–696
Noble B, Loh R, MacLennan S, Pesudovs K, Reynolds A, Bridges L, Burr J, Stewart O, Quereshi S (2004) Comparison of autologous serum eye drops with conventional therapy in a randomised crossover trial for ocular surface disease. Br J Ophthalmol 88:647-652
Noecker R (2001) Effects of common ophthalmic preservatives on ocular health. Adv Ther 18:205–215
Ogawa Y, Okamoto S, Mori T, Yamada M, Mashima Y, Watanabe R, Kuwana M, Tsubota K, Ikeda Y, Oguchi Y (2003) Autologous serum eye drops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Bone Marrow Transplant 31:579–583
Pfister RR, Renner ME (1978) The corneal and conjunctival surface in vitamin A deficiency: a scanning electron microscopy study. Invest Ophthalmol Vis Sci 17:874–883
Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC (1997) The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol 137:1445–1457
Poon AC, Geerling G, Dart JK, Fraenkel GE, Daniels JT (2001) Autologous serum eyedrops for dry eyes and epithelial defects: clinical and in vitro toxicity studies. Br J Ophthalmol 85:1188–1197
Rao RC, Varani J, Soong HK (1992) FGF promotes corneal stromal fibroblast motility. J Ocul Pharmacol 8:77–81
Rocha EM, Pelegrino FS, de Paiva CS, Vigorito AC, de Souza CA (2000) GVHD dry eyes treated with autologous serum tears. Bone Marrow Transplant 25:1101–1103
Rocha EM, Cunha DA, Carneiro EM, Boschero AC, Saad MJ, Velloso LA (2002) Insulin, insulin receptor and insulin-like growth factor-I receptor on the human ocular surface. Adv Exp Med Biol 506:607–610
Rodeck U, Jost M, Kari C, Shih DT, Lavker RM, Ewert DL, Jensen PJ (1997) EGF-R dependent regulation of keratinocyte survival. J Cell Sci 110 (Pt 2):113–121
Sommer A (1998) Xerophthalmia and vitamin A status. Prog Retin Eye Res 17:9–31
Spigelman AV, Deutsch TA, Sugar J (1987) Application of homologous fibronectin to persistent human corneal epithelial defects. Cornea 6:128–130
Tananuvat N, Daniell M, Sullivan LJ, Yi Q, McKelvie P, McCarty DJ, Taylor HR (2001) Controlled study of the use of autologous serum in dry eye patients. Cornea 20:802–806
Tripathi BJ, Tripathi RC (1989) Cytotoxic effects of benzalkonium chloride and chlorobutanol on human corneal epithelial cells in vitro. Lens Eye Toxic Res 6:395–403
Tsubota K, Satake Y, Ohyama M, Toda I, Takano Y, Ono M, Shinozaki N, Shimazaki J (1996) Surgical reconstruction of the ocular surface in advanced ocular cicatricial pemphigoid and Stevens–Johnson syndrome. Am J Ophthalmol 122:38–52
Tsubota K, Goto E, Fujita H, Ono M, Inoue H, Saito I, Shimmura S (1999) Treatment of dry eye by autologous serum application in Sjogren’s syndrome. Br J Ophthalmol 83:390–395
Tsubota K, Goto E, Shimmura S, Shimazaki J (1999) Treatment of persistent corneal epithelial defect by autologous serum application. Ophthalmology 106:1984–1989
Tsubota K, Higuchi A (2000) Serum application for the treatment of ocular surface disorders. Int Ophthalmol Clin 40:113–122
Ubels JL, Iorfino A, O’Brien WJ (1991) Retinoic acid decreases the number of EGF receptors in corneal epithelium and Chang conjunctival cells. Exp Eye Res 52:763–765
Vesaluoma M, Teppo AM, Gronhagen-Riska C, Tervo T (1997) Release of TGF-beta 1 and VEGF in tears following photorefractive keratectomy. Curr Eye Res 16:19–25
Wilson SE, Chen L, Mohan RR, Liang Q, Liu J (1999) Expression of HGF, KGF, EGF and receptor messenger RNAs following corneal epithelial wounding. Exp Eye Res 68:377–397
Zimmermann R, Jakubietz R, Jakubietz M, Strasser E, Schlegel A, Wiltfang J, Eckstein R (2001) Different preparation methods to obtain platelet components as a source of growth factors for local application. Transfusion 41:1217–1224
Acknowledgements
The authors thank Kaoru Araki-Sasaki, Tane Memorial Eye Hospital, Japan, and Fiona Stapleton, University of New South Wales, Australia, for providing HCE-T cell line; Gudrun Müller, Department of Ophthalmology, University of Lübeck, for technical assistance; Gudrun Knebel and Harry Manfeldt, Institute of Anatomy, University of Lübeck, for excellent support in SEM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, L., Hartwig, D., Harloff, S. et al. An optimised protocol for the production of autologous serum eyedrops. Graefe's Arch Clin Exp Ophthalmol 243, 706–714 (2005). https://doi.org/10.1007/s00417-004-1106-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-004-1106-5