Abstract
Background
Anecortave acetate is a synthetic derivative of cortisol, but very specific and irreversible chemical modifications to the cortisol structure have resulted in the creation of a potent inhibitor of blood vessel growth with no evidence non-clinically or clinically of glucocorticoid receptor-mediated bioactivity. The clinical safety of Anecortave Acetate administered as a posterior juxtascleral depot every 6 months for up to 4 years is reviewed in this manuscript.
Methods
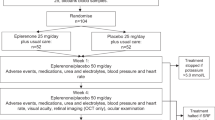
Clinical safety and efficacy of the novel angiostatic agent Anecortave Acetate for Depot Suspension was evaluated in patients with subfoveal exudative age-related macular degeneration (AMD) in a masked, randomized, dose-duration clinical trial completed in June 2003. This safety and efficacy study enrolled and treated 128 patients at 18 clinical sites in the US and EU. This was the first clinical trial of Anecortave Acetate for Depot Suspension administered as a posterior juxtascleral depot. Assessments of clinical safety were made with general physical examinations including electrocardiograms and hematology/serum chemistry/urinalysis, detailed ophthalmic evaluations with fluorescein/indocyanine green angiography and assessments of best-corrected logMAR visual acuity. All safety reports have been reviewed periodically by an Independent Safety Committee responsible for overseeing these activities.
Results
No clinically relevant safety issues related to either Anecortave Acetate for Depot Suspension or the administration procedure have been identified by an Independent Safety Committee. The most frequent safety issues reported were cataractous changes, decreased visual acuity, ptosis, ocular pain, abnormal vision and subconjunctival hemorrhage, but the majority of these were assessed as unrelated to treatment.
Conclusions
Anecortave Acetate for Depot Suspension (3, 15 and 30 mg) is clinically safe following administration and re-administration at 6-month intervals as a posterior juxtascleral depot using a specially designed curved cannula.
Similar content being viewed by others
References
Anecortave Acetate Clinical Study Group (2003) Anecortave acetate as monotherapy for the treatment of subfoveal lesions in patients with exudative age-related macular degeneration (AMD): interim (month 6) analysis of clinical safety and efficacy. Retina 23:14–23
Anecortave Acetate Clinical Study Group (2003) Anecortave acetate as monotherapy for treatment of subfoveal neovascularization in age-related macular degeneration: the twelve-month clinical outcomes. Ophthalmology 110:2372–2383
BenEzra D, Griffin BW, Maftzir G, Sharif NA, Clark AF (1997) Topical formulations of novel angiostatic steroids inhibit rabbit corneal neovascularization. Invest Ophthalmol Vis Sci 38:1954–1962
Clark AF (1997) AL-3789: a novel ophthalmic angiostatic steroid. Expert Opin Investig Drugs 6:1867–1877
Clark AF, Mellon J, Li X-Y, Ma D, Lieber H, Apte R, Alizadeh H, Hegde S, McLenaghan A, Mayhew E, D’Orazio TJ, Niederkorn JY (1999) Inhibition of intraocular tumor growth by topical application of the angiostatic steroid anecortave acetate. Invest Ophthalmol Vis Sci 40:2158–2162
Cochereau-Massin I, Lehoang P, Lautier-Frau M, Zazoun L, Marcel P, Robinet M, Matheron S, Katlama C, Gharakhanian S, Rozenbaum W, Ingrand D, Gentilini M (1991) Efficacy and tolerance of intravitreal ganciclovir in cytomegalovirus retinitis in acquired immune deficiency syndrome. Ophthalmology 98:1348–1355
Heinemann M-H (1989) Long-term intravitreal ganciclovir therapy for cytomegalovirus retinopathy. Arch Ophthalmol 107:1767–1772
McNatt LG, Weimer L, Yanni J, Clark AF (1999) Angiostatic activity of steroids in the chick embryo CAM and rabbit corneal pocket models of neovascularization. J Ocul Pharmacol 15:413–423
Nelson ML, Tennant MTS, Sivalingam A, Regillo CD, Belmont J, Martidis A (2003) Infectious and presumed noninfectious endophthalmitis after intravitreal triamcinolone acetonide injection. Retina 23:686–691
Penn JS, Rajaratnam VS, Collier RJ, Clark AF (2001) The effect of an angiostatic steroid on neovascularization in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci 42:283–290
Acknowledgements
Clinical trial activities funded by Alcon Research, Ltd., Fort Worth, TX. Part of this study was presented on the Meeting of the Deutsche Ophthalmologische Gesellschaft, September 2003, Berlin.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Independent Safety Committee
The committee comprises the following members:
-
Donald J. D’Amico, MD, Chairman, Massachusetts Eye & Ear Infirmary, Boston, MA
-
Carl Regillo, MD, Wills Eye Hospital, Philadelphia, PA
-
William F. Mieler, MD, Cullen Eye Institute, Houston, TX
-
Cary Schneebaum, MD, New York, NY
-
Cliff Beasley, MD, Fort Worth, TX, Alcon Research, Ltd. Medical Monitor
Rights and permissions
About this article
Cite this article
Augustin, A.J., D’Amico, D.J., Mieler, W.F. et al. Safety of posterior juxtascleral depot administration of the angiostatic cortisene anecortave acetate for treatment of subfoveal choroidal neovascularization in patients with age-related macular degeneration. Graefe's Arch Clin Exp Ophthalmol 243, 9–12 (2005). https://doi.org/10.1007/s00417-004-0961-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-004-0961-4