Abstract
Background
Cultured retinal pigment epithelial (RPE) cells exposed to vitreous lose their cuboidal epithelial phenotype and transform to a fibroblast-like morphology. This study investigates whether the loss of RPE phenotype will affect the ability of RPE to phagocytize photoreceptor outer segments (ROS).
Methods
First-passage porcine RPE cells were grown to confluence and exposed to an equal mixture of freshly homogenized vitreous and DMEM (10% FBS) for 48 h to allow transformation to occur. The phagocytic ability of normal epithelial and vitreous-transformed RPE cultures was evaluated by determining the number of ROS bound and ingested. The expression and distribution of αvβ5 integrin in control and vitreous-treated RPE were examined by immunoprecipitation coupled with immunoblotting and immunohistochemistry and the contribution of αvβ5 integrin to ROS phagocytosis was evaluated using a functional blocking antibody.
Results
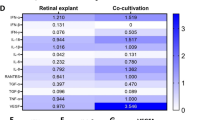
Bound ROS on vitreous-transformed RPE decreased to 33% of control and ingested ROS decreased to 47%. The expression of αvβ5 integrin was decreased significantly following vitreous transformation as compared with controls and the immunolocalization of αvβ5 on control RPE was restricted to apical patches while on vitreous-treated RPE, labeling was diminished and randomly dispersed. Five times less blocking antibody was required to attain maximal phagocytic inhibition in these cultures, but some phagocytic activity remained in both normal and transformed cultures even at saturating concentrations.
Conclusion
The decrease in ROS phagocytosis observed in transformed RPE correlated with the down- regulation of αvβ5 integrin expression and its random distribution on the RPE.






Similar content being viewed by others
References
Anderson DH, Guerin CJ, Matsumoto B, Pfeffer BA (1990) Identification and localization of a beta-1 receptor from the integrin family in mammalian retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 31:81–93
Anderson DH, Johnson LV, Hageman GS (1995) Vitronectin receptor expression and distribution at the photoreceptor-retinal pigment epithelial interface. J Comp Neurol 360:1–16
Bodary SC, McLean JW (1990) The integrin β1 subunit associates with the vitronectin receptor αv subunit to form a novel vitronectin receptor in a human embryonic kidney cell line. J Biol Chem 265:5938–5941
Boyle D, Tien LF, Cooper NG, Shepherd V, McLaughlin BJ (1991) A mannose receptor is involved in retinal phagocytosis. Invest Ophthalmol Vis Sci 32:1464–1470
Busk M, Pytela R, Sheppard D (1992) Characterization of the integrin αvβ6 as a fibronectin-binding protein. J Biol Chem 267:5790–5796
Chaitin MH, Hall MO (1983) Defective ingestion of rod outer segments by cultured dystrophic rat pigment epithelial cells. Invest Ophthalmol Vis Sci 24:812–820
D'Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D (2000) Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Human Mol Gen 9:645–651
Edwards RB (1991) Stimulation of rod outer segment phagocytosis by serum occurs only at the RPE apical surface. Exp Eye Res 53:229–232
Edwards RB, Flaherty PM (1986) Increased phagocytosis of outer segments in the presence of serum by cultured normal, but not dystrophic, rat retinal pigment epithelium. J Cell Physiol 127:293–296
Feng W, Yasamura D, Matthes MT, LaVail MM, Vollrath D (2002) Mertk triggers uptake of photoreptor outer segments during phagocytosis by cultured retinal pigment epithelial cells. J Biol Chem 277:17016–17022
Finnemann SC, Bonilha VL, Marmorstein AD, Rodriguez-Boulan E (1997). Phagocytosis of rod outer segments by retinal pigment epithelial cells requires αvβ5 integrin for binding but not for internalization. Proc Natl Acad Sci USA 94:12932–12937
Freed E, Gailit J, van der Geer P, Ruoslahti E, Hunter T (1989) A novel integrin β subunit is associated with the vitronectin receptor α subunit (αv) in a human osteosarcoma cell line and is a substrate for protein kinase C. EMBO J 8:2955–2965
Gregory CY, Converse CA, Foulds WS (1990) Effect of glycoconjugates on rod outer segment phagocytosis by retinal pigment epithelial explants in vitro assessed by a specific double radioimmunoassay procedure. Curr Eye Res 9:65–77
Grisanti S, Guidry C (1995) Transdifferentiation of retinal pigment epithelial cells from epithelial to mesenchymal phenotype. Invest Ophthalmol Vis Sci 36:391–405
Hall MO, Abrams T (1987) Kinetic studies of rod outer segment binding and ingestion by cultured rat RPE cells. Exp Eye Res 45:907–922
Hall MO, Burgess BL, Abrams TA (1997) Identification and cloning of a 55 kDa RPE plasma membrane protein: a putative receptor for OS phagocytosis. Invest Ophthalmol Vis Sci 38:s1166
Hall MO, Burgess BL, Abrams TA, Ershov AV, Gregory CY (1996) Further studies on the identification of the phagocytosis receptor of rat retinal pigment epithelial cells. Exp Eye Res 63:255–264
Hall MO, Burgess BL, Abrams TA (1998) Molecular studies of a candidate receptor for outer segment phagocytosis. Exp Eye Res 67 [Suppl 1]:536
Hall MO, Prieto AL, Obin MS, Abrams TA, Burgess BL, Heeb MJ, Agnew BJ (2001) Outer segment phagocytosis by cultured retinal pigment epithelial cells requires Gas6. Exp Eye Res 73:509–520
Ho T-C, Del Priore LV (1997) Reattachment of cultured human retinal pigment epithelium to extracellular matrix and human Bruch's membrane. Invest Ophthalmol Vis Sci 38:1110 1118
Kirchhof B, Kirchhof E, Ryan SJ, Sorgente N (1988) Human retinal pigment epithelial cell cultures: phenotypic modulation by vitreous and macrophages. Exp Eye Res 47:457–463
Lin H, Clegg DO (1998) Integrin αvβ5 participates in the binding of photoreceptor rod outer segments during phagocytosis by cultured human retinal pigment epithelium. Invest Ophthalmol Vis Sci 39:1703–1712
Lutz DA, McLaughlin BJ (1995) Down-regulation and redistribution of adherens and occludens junction components during vitreous-induced RPE transformation. Invest Ophthalmol Vis Sci 36:s752
Machemer R, Laqua H (1975) Pigment epithelium proliferation in retinal detachment (massive periretinal proliferation). Am J Ophthalmol 80:1–23
Machemer R, van Harn D, Aaberg TM (1978) Pigment epithelial proliferation in human retinal detachment with massive periretinal proliferation. Am J Ophthalmol 85:181–191
Mayerson PL, Hall MO (1986) Rat retinal pigment epithelial cells show specificity of phagocytosis in vitro. J Cell Biol 103:299–308
McKay BS, Burke JM (1994) Separation of phenotypically distinct subpopulations of cultured human retinal pigment epithelial cells. Exp Cell Res 213:85–92
McKay BS, Irving PE, Skumatz CM, Burke JM (1997) Cell-cell adhesion molecules and the development of an epithelial phenotype in cultured human retinal pigment epithelial cells. Exp Eye Res 65:661–671
McLaren MJ (1996) Kinetics of rod outer segment phagocytosis by cultured retinal pigment epithelial cells. Relationship to cell morphology. Invest Ophthalmol Vis Sci 37:1213–1224
Miceli MV, Newsome DA (1996) Effects of extracellular matrix and Bruch's membrane on retinal outer segment phagocytosis by cultured human retinal pigment epithelium. Curr Eye Res 15:17–26
Miceli MV, Newsome DA, Tate DJ, Jr (1997) Vitronectin is responsible for serum- stimulated uptake of rod outer segments by cultured retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 38:1588–1597
Nandrot E, Dufour EM, Provost AC, Pequignot MO, Bonnel S, Gogat K, Marchant D, Rouillac C, Sepulchre de Conde B, Bihoreau M-T, Shaver C, Dufier J-L, Marsac C, Lathrop M, Menasche M, Abitbol MM (2000) Homozygous deletion in the coding sequence of the c-mer gene in the RCS rat unravels general mechanisms of physiological cell adhesion and apoptosis. Neurobiol Dis 7:586–599
Nishimura SL, Boylen KP, Einheber S, Milner TA, Ramos DM, Pytela R (1998) Synaptic and glial localization of the integrin αvβ8 in mouse and rat brain. Brain Res 791:271–282
Panetti TS, McKeown-Longo PJ (1993) The αvβ5 integrin receptor regulates receptor-mediated endocytosis of vitronectin. J Biol Chem 268:11492–11495
Ryeom SW, Silverstein RL, Scotto A, Sparrow JR (1996) Binding of anionic phospholipids to retinal pigment epithelium may be mediated by the scavenger receptor CD36. J Biol Chem 271:20536–20539
Ryeom SW, Sparrow JR, Silverstein RL (1996) CD36 participates in the phagocytosis of rod outer segments by retinal pigment epithelium. J Cell Sci 109:387-395
Savill J, Dransfield I, Hogg N, Haslett C (1990) Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature 343:170–173
Savill J, Hogg N, Ren Y, Haslett C (1992) Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest 90:1513–1522
Sebag J (1992) The vitreous. In: Hart WM Jr (ed) Adler's Physiology of the eye. Mosby Year Book, St. Louis, pp 268–347
Shepherd VL, Tarnowski BI, McLaughlin BJ (1991) Isolation and characterization of a mannose receptor from human pigment epithelium. Invest Ophthalmol Vis Sci 32:1779–1784
Smith JW, Vestal DJ, Irwin SV, Burke TA, Cheresh DA (1990) Purification and functional characterization of integrin αvβ5. An adhesion receptor for vitronectin. J Biol Chem 265:11008–11013
Vollrath D, Feng W, Duncan JL, Yasamura D, D'Cruz PM, Chappelow A, Matthes MT, Kay MA, LaVail MM (2001) Correction of the retinal dystrophy phenotype of the RCS rat by viral gene transfer of Mertk. Proc Natl Acad Sci 98:12584–12589
Young RW, Bok D (1969) Participation of the retinal pigment epithelium in the rod outer segment renewal process J Cell Biol 42:392–403
Acknowledgements
We are grateful to Dr. Nigel G. F. Cooper for helpful discussions and comments. This research was supported by NIH grants EY 02853 (B.J.M.), EY 11130 (D.A.L.) and an unrestricted grant from Research to Prevent Blindness.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, W., Zheng, J.J., Lutz, D.A. et al. Loss of RPE phenotype affects phagocytic function. Graefe's Arch Clin Exp Ophthalmol 241, 232–240 (2003). https://doi.org/10.1007/s00417-002-0617-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-002-0617-1