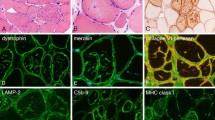
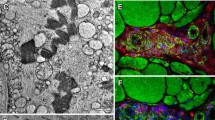
Abstract
Objective
X-linked myopathy with excessive autophagy (XMEA) linked to the VMA21 gene leads to autophagy failure with progressive vacuolation and atrophy of skeletal muscles. Current knowledge of this rare disease is limited. Our objective was to define the clinical, radiological, and natural history of XMEA.
Methods
We conducted a retrospective study collecting clinical, genetic, muscle imaging, and biopsy data of XMEA patients followed in France and reviewed the literature for additional cases.
Results
Eighteen males had genetically confirmed XMEA in France, carrying four different VMA21 variants. Mean age at disease onset was 9.4 ± 9.9 (range 1–40) years. In 14/18 patients (77.8%), onset occurred during childhood (< 15 years); however in four patients, the disease started in adulthood. Patients had anterior and medial compartment thigh muscle weakness, distal contractures (56.3%), elevated CK levels (1287.9 ± 757.8 U/l) and autophagic vacuoles with sarcolemmal features on muscle histopathology. Muscle MRI (n = 10) showed a characteristic pattern of lower limb muscle involvement. In 11 patients, outcome measures were available for an average follow-up period of 10.6 ± 9.8 years and six of them show disease progression. Mean change of functional outcomes was 0.5 ± 1.2 points for Brooke and 2.2 ± 2.5 points for Vignos score, 7/16 patients (43.8%) needed a walking aid and 3/16 (18.8%) were wheelchair-bound (median age of 40 years old, range 39–48). The variant c.164-7 T > G was associated with a later onset of symptoms. Respiratory insufficiency was common (57.1%) but cardiac involvement rare (12.5%).
Interpretation
XMEA has variable age of onset, but a characteristic clinical, histopathological, and muscle imaging presentation, guiding the diagnosis. Although slowly, motor disability progresses with time, and relevant genotype–phenotype correlations will help design future clinical trials.




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ramachandran N, Munteanu I, Wang P et al (2013) VMA21 deficiency prevents vacuolar ATPase assembly and causes autophagic vacuolar myopathy. Acta Neuropathol 125(3):439–457
Sugie K, Noguchi S, Kozuka Y et al (2005) Autophagic vacuoles with sarcolemmal features delineate danon disease and related myopathies. J Neuropathol Exp Neurol 64(6):513–522
Kalimo H, Savontaus M-L, Lang H et al (1988) X-Linked myopathy with excessive autophagy: a new hereditary muscle disease. Ann Neurol 23(3):258–265
Nemazanyy I, Blaauw B, Paolini C et al (2013) Defects of Vps15 in skeletal muscles lead to autophagic vacuolar myopathy and lysosomal disease. EMBO Mol Med 5(6):870–890
Mercuri E, Pichiecchio A, Allsop J et al (2007) Muscle MRI in inherited neuromuscular disorders: past present, and future. J Magn Reson Imag 440:433–440
Richards S, Aziz N, Bale S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of medical genetics and genomics and the association for molecular pathology. Genet Med 17(5):405–424
Villanova M, Louboutin JP, Chateau D et al (1995) X-linked vacuolated myopathy: complement membrane attack complex on surface membrane of injured muscle fibers. Ann Neurol 37(5):637–645
Chabrol B, Figarella-Branger D, Coquet M et al (2001) X-linked myopathy with excessive autophagy: a clinicopathological study of five new families. Neuromuscul Disord 11(4):376–388
Kurashige T, Takahashi T, Yamazaki Y et al (2013) Elevated urinary β2 microglobulin in the first identified Japanese family afflicted by X-linked myopathy with excessive autophagy. Neuromuscul Disord 23(11):911–916
Crockett CD, Ruggieri A, Gujrati M et al (2014) Late adult-onset of X-linked myopathy with excessive autophagy. Muscle Nerve 50(1):138–144
Ruggieri A, Ramachandran N, Wang P et al (2015) Non-coding VMA21 deletions cause X-linked myopathy with excessive autophagy. Neuromuscul Disord 25(3):207–211
Mercier S, Magot A, Caillon F et al (2015) Muscle magnetic resonance imaging abnormalities in X-linked myopathy with excessive autophagy. Muscle Nerve 52(4):673–680
Rao S, Chandra S, Narayanappa G (2019) X-linked myopathy with excessive autophagy; a case report. Neurol India 67(5):1344
Cotta A, Carvalho E, da Cunha-Junior AL et al (2020) Clinical, imaging, morphologic, and molecular features of X-linked VMA21-related myopathy in two unrelated Brazilian families. J Neurol Sci 415:116977
Alon T, Sadeh M, Lev D, Dabby R (2021) X-linked myopathy with excessive autophagy: first report of an Israeli family presenting with late onset lower limb girdle weakness. Neuromuscul Disord 31(9):854–858
Yang J, Chen D, Feng L et al (2022) X-linked myopathy with excessive autophagy due to a mutation in VMA21 gene: the first case in China. Neurol Sci 43(3):2137–2139
Pegat A, Streichenberger N, Lacoste N et al (2022) Novel intronic mutation in VMA21 causing severe phenotype of X-linked myopathy with excessive autophagy—case report. Genes (Basel) 13(12):2245
Ishizaki M, Kobayashi M, Adachi K et al (2018) Female dystrophinopathy: review of current literature. Neuromuscul Disord 28(7):572–581
Kishnani PS, Hwu WL, Mandel H et al (2006) A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr 148(5):671-676.e2
Sugie K, Yamamoto A, Murayama K et al (2002) Clinicopathological features of genetically confirmed Danon disease. Neurology 58(12):1773–1778
Cocchiararo I, Cattaneo O, Rajendran J et al (2023) Identification of a muscle-specific isoform of VMA21 as a potent actor in X-linked myopathy with excessive autophagy pathogenesis. Hum Mol Genet 26(3):312–318
Hobson-Webb LD, DeArmey S, Kishnani PS (2011) The clinical and electrodiagnostic characteristics of Pompe disease with post-enzyme replacement therapy findings. Clin Neurophysiol 122(11):2312–2317
Dowling JJ, Moore SA, Kalimo H, Minassian BA (2015) X-linked myopathy with excessive autophagy: a failure of self-eating. Acta Neuropathol 129(3):383–390
Tasca G, Iannaccone E, Monforte M et al (2012) Muscle MRI in becker muscular dystrophy. Neuromuscul Disord 22(SUPPL. 2):S100–S106
Fischer D, Hafner P, Rubino D et al (2016) The 6-minute walk test, motor function measure and quantitative thigh muscle MRI in Becker muscular dystrophy: a cross-sectional study. Neuromuscul Disord 26(7):414–422
Munteanu I, Ramachandran N, Ruggieri A et al (2015) Congenital autophagic vacuolar myopathy is allelic to X-linked myopathy with excessive autophagy. Neurology 84(16):1714–1716
Acknowledgements
The authors would like to thank Frédéric Fer for assistance with developing the R code to generate a heatmap of the MRI muscle involvement.
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors. MS is supported by a clinician scientist INSERM position (CIHU INSERM).
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors. MS is supported by a clinician scientist INSERM position (CIHU INSERM).
Author information
Authors and Affiliations
Contributions
Concept and design: GFE, AB, TS. Acquisition and analysis of data: GFE, GA, MS, IAB, FD, GS, FC, SM, YP, AM, AP, ESC, BC, SG, MK, VB, TE. Drafting of the manuscript and figures: GFE, GA, MS, AB, CM, TS.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fernández-Eulate, G., Alfieri, G., Spinazzi, M. et al. Phenotype variability and natural history of X-linked myopathy with excessive autophagy. J Neurol (2024). https://doi.org/10.1007/s00415-024-12298-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00415-024-12298-0