Abstract
Background
one of the most important therapeutic goals in relapse-onset multiple sclerosis is to preclude conversion to secondary progression. Our objective was to determine the risk of progression associated with natalizumab treatment in an registry-based cohort of patients and to identify determinant factors.
Methods
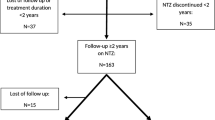
Patients with relapse-onset multiple sclerosis from the Registre Lorrain des Scléroses en Plaques (ReLSEP) were included if they had received one infusion of natalizumab between 2002 and 2021. The risk of secondary progression was determined using a standardized definition and a multi-state estimator to account for the possibility of stopping natalizumab before progression, and analyzed with multivariate Cox models.
Results
574 patients were followed up for a median of 6.7 years. Of the 304 who stopped NTZ before progression onset, the probabilities (95% confidence interval) to convert to progression after 1, 2, 5 and 10 years were 3.2% (2.0–4.8%), 5.3% (3.6–7.3%), 17.5% (14.3–21.3%) and 28.3% (23.7–33.7%), respectively. Discontinuation of NTZ during follow-up was significantly associated with an increased risk of conversion in case of no resumption of a highly active treatment thereafter (adjusted hazard ratio = 2.7; 95% confidence interval 1.5–4.9; p = 0.001). The use of such a treatment was associated with a lower risk of progression (adjusted hazard ratio = 0.42; 95% confidence interval 0.23–0.79; p = 0.007).
Conclusion
the risk of conversion to secondary progression associated with natalizumab treatment is relatively low but increases in case of natalizumab discontinuation in the absence of switch to highly active immunosuppressant.

Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author, GM. The data are not publicly available because they contain information that could compromise the privacy of research participants.
References
Weinshenker BG, Bass B, Rice GP et al (1989) The natural history of multiple sclerosis: a geographically based study. I Clin Course Disabil Brain 112(Pt 1):133–146
Tremlett H, Zhao Y, Devonshire V (2008) Natural history of secondary-progressive multiple sclerosis. Mult Scler 14:314–324. https://doi.org/10.1177/1352458507084264
Scalfari A, Neuhaus A, Daumer M et al (2014) Onset of secondary progressive phase and long-term evolution of multiple sclerosis. J Neurol Neurosurg Psychiatry 85:67–75. https://doi.org/10.1136/jnnp-2012-304333
Brown JWL, Coles A, Horakova D et al (2019) Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA 321:175–187. https://doi.org/10.1001/jama.2018.20588
Polman CH, Miller DH, Wajgt A, Sandrock AW (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 12:899
Rudick RA, Stuart WH, Calabresi PA et al (2006) Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 354:911–923. https://doi.org/10.1056/NEJMoa044396
Zivadinov R, Hojnacki D, Bergsland N et al (2016) Effect of natalizumab on brain atrophy and disability progression in multiple sclerosis patients over 5 years. Eur J Neurol 23:1101–1109. https://doi.org/10.1111/ene.12992
Graf J, Leussink VI, Soncin G et al (2021) Relapse-independent multiple sclerosis progression under natalizumab. Brain Commun 3:fcab229. https://doi.org/10.1093/braincomms/fcab229
Butzkueven H, Kappos L, Wiendl H et al (2020) Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP). J Neurol Neurosurg Psychiatry 91:660–668. https://doi.org/10.1136/jnnp-2019-322326
Rae-Grant A, Day GS, Marrie RA et al (2018) Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the american academy of neurology. Neurology 90:777–788. https://doi.org/10.1212/WNL.0000000000005347
Debouverie M, Pittion-Vouyovitch S, Louis S et al (2008) Natural history of multiple sclerosis in a population-based cohort. Eur J Neurol 15:916–921. https://doi.org/10.1111/j.1468-1331.2008.02241.x
El Adssi H, Debouverie M, Guillemin F, LORSEP Group (2012) Estimating the prevalence and incidence of multiple sclerosis in the Lorraine region, France, by the capture-recapture method. Mult Scler 18:1244–1250. https://doi.org/10.1177/1352458512437811
Thompson AJ, Banwell BL, Barkhof F et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17:162–173. https://doi.org/10.1016/S1474-4422(17)30470-2
Poser CM, Paty DW, Scheinberg L et al (1983) New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 13:227–231. https://doi.org/10.1002/ana.410130302
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33:1444–1444. https://doi.org/10.1212/WNL.33.11.1444
Confavreux C, Compston DA, Hommes OR et al (1992) EDMUS, a European database for multiple sclerosis. J Neurol Neurosurg Psychiatry 55:671–676. https://doi.org/10.1136/jnnp.55.8.671
Lorscheider J, Buzzard K, Jokubaitis V et al (2016) Defining secondary progressive multiple sclerosis. Brain 139:2395–2405. https://doi.org/10.1093/brain/aww173
Katz Sand I, Krieger S, Farrell C, Miller AE (2014) Diagnostic uncertainty during the transition to secondary progressive multiple sclerosis. Mult Scler 20:1654–1657. https://doi.org/10.1177/1352458514521517
Davies F, Wood F, Brain KE et al (2016) The transition to secondary progressive multiple sclerosis: an exploratory qualitative study of health professionals’ experiences. Int J MS Care 18:257–264. https://doi.org/10.7224/1537-2073.2015-062
Aalen O (1978) Nonparametric estimation of partial transition probabilities in multiple decrement models. Ann Stat 6:534–545. https://doi.org/10.1214/aos/1176344198
Therneau TM, Grambsch PM, Fleming TR (1990) Martingale-based residuals for survival models. Biometrika 77:147–160. https://doi.org/10.1093/biomet/77.1.147
Eilers PHC, Marx BD (1996) Flexible smoothing with B-splines and penalties. Stat Sci 11:89–121. https://doi.org/10.1214/ss/1038425655
Venables WN, Ripley BD (2002) Modern Applied Statistics with S. Springer, New York, NY
Auer M, Zinganell A, Hegen H et al (2021) Experiences in treatment of multiple sclerosis with natalizumab from a real-life cohort over 15 years. Sci Rep 11:23317. https://doi.org/10.1038/s41598-021-02665-6
Bigaut K, Fabacher T, Kremer L et al (2021) Long-term effect of natalizumab in patients with RRMS: TYSTEN cohort. Mult Scler 27:729–741. https://doi.org/10.1177/1352458520936239
Sorensen PS, Koch-Henriksen N, Petersen T et al (2014) Recurrence or rebound of clinical relapses after discontinuation of natalizumab therapy in highly active MS patients. J Neurol 261:1170–1177. https://doi.org/10.1007/s00415-014-7325-8
Prosperini L, Kinkel RP, Miravalle AA et al (2019) Post-natalizumab disease reactivation in multiple sclerosis: systematic review and meta-analysis. Ther Adv Neurol Disord 12:1756286419837809. https://doi.org/10.1177/1756286419837809
Roos I, Malpas C, Leray E et al (2022) Disease reactivation after cessation of disease-modifying therapy in patients with relapsing-remitting multiple sclerosis. Neurology 99:e1926–e1944. https://doi.org/10.1212/WNL.0000000000201029
Prosperini L, Annovazzi P, Capobianco M et al (2015) Natalizumab discontinuation in patients with multiple sclerosis: profiling risk and benefits at therapeutic crossroads. Mult Scler 21:1713–1722. https://doi.org/10.1177/1352458515570768
Hellwig K, Tokic M, Thiel S et al (2022) Multiple sclerosis disease activity and disability following discontinuation of natalizumab for pregnancy. JAMA Netw Open 5:e2144750. https://doi.org/10.1001/jamanetworkopen.2021.44750
Stankoff B, Mrejen S, Tourbah A et al (2007) Age at onset determines the occurrence of the progressive phase of multiple sclerosis. Neurology 68:779–781. https://doi.org/10.1212/01.wnl.0000256732.36565.4a
Scalfari A, Neuhaus A, Daumer M et al (2011) Age and disability accumulation in multiple sclerosis. Neurology 77:1246–1252. https://doi.org/10.1212/WNL.0b013e318230a17d
Confavreux C, Vukusic S (2006) Natural history of multiple sclerosis: a unifying concept. Brain 129:606–616. https://doi.org/10.1093/brain/awl007
Acknowledgements
The authors thank the patients, the research nurses, clinical coordinators, technicians and statistics team for their contributions to patient care and conduct of the protocol.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
LS, JE, MS, SPV, MD and GM declare that there is no conflict of interest in line with this work. FG reports grants from Roche, Merck and Biogen to his institution.
Ethical statement
All patients gave their informed consent to participate in epidemiological research. Data collection was approved by the French National Commission for Data Protection and Liberties (CNIL n° 913,001), and confidentiality and safety of the data were ensured in accordance with their recommendations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Scherer, L., Soudant, M., Pittion-Vouyovitch, S. et al. Risk of secondary progression in patients with highly active multiple sclerosis treated with natalizumab: a real-life study. J Neurol 271, 2216–2224 (2024). https://doi.org/10.1007/s00415-024-12266-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-024-12266-8