Abstract
Background
Active relapsing–remitting (RR) and secondary progressive (SP) multiple sclerosis (MS) are currently defined as “relapsing MS” (RMS). The aim of this cross-sectional study was to assess drivers of treatment switches due to clinical relapses in a population of RMS patients collected in the Italian MS and Related Disorders Register (I-MS&RD).
Methods
RRMS and SPMS patients with at least one relapse in a time window of 2 years before of data extraction were defined as RMS. Factors associated with disease-modifying therapy (DMT) switching due to clinical activity were assessed through multivariable logistic regression models in which treatment exposure was included as the last recorded DMT and the last DMT’s class [moderate-efficacy (ME), high-efficacy (HE) DMTs and anti-CD20 drugs].
Results
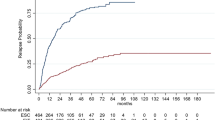
A cohort of 4739 RMS patients (4161 RRMS, 578 SPMS) was extracted from the I-MS&RD. A total of 2694 patients switching DMTs due to relapses were identified. Switchers were significantly (p < 0.0001) younger, less disabled, more frequently affected by an RR disease course in comparison to non-switcher patients. The multivariable logistic regression models showed that Alemtuzumab (OR 0.08, 95% CI 0.02–0.37), Natalizumab (0.48, 0.30–0.76), Ocrelizumab (0.1, 0.02–0.45) and Rituximab (0.23, 0.06–0.82) exposure was a protective factor against treatment switch due to relapses. Moreover, the use of HE DMTs (0.43, 0.31–0.59), especially anti-CD20 drugs (0.14, 0.05–0.37), resulted to be a protective factor against treatment switch due to relapses in comparison with ME DMTs.
Conclusions
More than 50% of RMS switched therapy due to disease activity. HE DMTs, especially anti-CD20 drugs, significantly reduce the risk of treatment switch.
Similar content being viewed by others
References
Filippi M, Danesi R, Derfuss T et al (2022) Early and unrestricted access to high-efficacy disease-modifying therapies: a consensus to optimize benefits for people living with multiple sclerosis. J Neurol 269(3):1670–1677. https://doi.org/10.1007/s00415-021-10836-8
European Medicines Agency. Guideline on clinical investigation of medicinal products for the treatment of Multiple Sclerosis. EMA/CHMP/771815/2011, https://www.ema.europa.eu/en/documents/scientific-guideline/guidelineclinical-investigation-medicinal-productstreatment-multiple-sclerosis_en-0.pdf
Bayas A, Christ M, Faissner S et al (2023) Disease-modifying therapies for relapsing/active secondary progressive multiple sclerosis—a review of population-specific evidence from randomized clinical trials. Ther Adv Neurol Disord. https://doi.org/10.1177/17562864221146836. (Published 2023 Jan 24)
Gross RH, Corboy JR (2019) Monitoring, switching, and stopping multiple sclerosis disease-modifying therapies [published correction appears in Continuum (Minneap Minn). 2019 Aug;25(4):1175]. Continuum (Minneap Minn). 25(3):715–735. https://doi.org/10.1212/CON.00000000000007383
Ziemssen T, Derfuss T, de Stefano N et al (2016) Optimizing treatment success in multiple sclerosis. J Neurol 263(6):1053–1065. https://doi.org/10.1007/s00415-015-7986-y
He A, Merkel B, Brown JWL et al (2020) Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol 19(4):307–316. https://doi.org/10.1016/S1474-4422(20)30067-3
Harding K, Williams O, Willis M et al (2019) Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol 76(5):536–541. https://doi.org/10.1001/jamaneurol.2018.4905
Merkel B, Butzkueven H, Traboulsee AL, Havrdova E, Kalincik T (2017) Timing of high-efficacy therapy in relapsing–remitting multiple sclerosis: A systematic review. Autoimmun Rev 16(6):658–665. https://doi.org/10.1016/j.autrev.2017.04.010
Hauser SL, Cree BAC (2020) Treatment of multiple sclerosis: a review. Am J Med 133(12):1380-1390.e2. https://doi.org/10.1016/j.amjmed.2020.05.049
Iaffaldano P, Lucisano G, Butzkueven H et al (2021) Early treatment delays long-term disability accrual in RRMS: results from the BMSD network. Mult Scler 27(10):1543–1555. https://doi.org/10.1177/13524585211010128
Trojano M, Bergamaschi R, Amato MP et al (2019) The Italian multiple sclerosis register [published correction appears in Neurol Sci. 2019 Apr;40(4):907]. Neurol Sci 40(1):155–165. https://doi.org/10.1007/s10072-018-3610-0
Magyari M, Joensen H, Laursen B, Koch-Henriksen N (2021) The Danish Multiple Sclerosis Registry. Brain Behav 11(1):e01921. https://doi.org/10.1002/brb3.1921
Trojano M, Kalincik T, Iaffaldano P, Amato MP (2022) Interrogating large multiple sclerosis registries and databases: what information can be gained? Curr Opin Neurol 35(3):271–277. https://doi.org/10.1097/WCO.0000000000001057
Lorscheider J, Buzzard K, Jokubaitis V et al (2016) Defining secondary progressive multiple sclerosis. Brain 139(Pt 9):2395–2405. https://doi.org/10.1093/brain/aww173
Iaffaldano P, Lucisano G, Patti F et al (2021) Transition to secondary progression in relapsing-onset multiple sclerosis: definitions and risk factors. Mult Scler 27(3):430–438. https://doi.org/10.1177/1352458520974366
https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2023-03-27&atto.codiceRedazionale=23A01958&elenco30giorni=false. Accessed 16 Nov 2023
https://www.iss.it/documents/20126/8331678/LG-340-SIN_SM.pdf/b3801eb6-2662-37b6-faeb-3612dd99364c?t=1677495451891. Accessed 16 Nov 2023
Scott TF, Diehl D, Elmalik W, Gettings EJ, Hackett C, Schramke CJ (2019) Multiple sclerosis relapses contribute to long-term disability. Acta Neurol Scand 140(5):336–341. https://doi.org/10.1111/ane.13149
Jokubaitis VG, Spelman T, Kalincik T et al (2016) Predictors of long-term disability accrual in relapse-onset multiple sclerosis. Ann Neurol 80(1):89–100. https://doi.org/10.1002/ana.24682
Lublin FD, Häring DA, Ganjgahi H et al (2022) How patients with multiple sclerosis acquire disability. Brain 145(9):3147–3161. https://doi.org/10.1093/brain/awac016
Rotstein D, Montalban X (2019) Reaching an evidence-based prognosis for personalized treatment of multiple sclerosis. Nat Rev Neurol 15(5):287–300. https://doi.org/10.1038/s41582-019-0170-8
Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D et al (2018) ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler 24(2):96–120
Rae-Grant A, Day GS, Marrie RA, Rabinstein A, Cree BAC, Gronseth GS et al (2018) Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology 90(17):777–788
Patti F, Chisari CG, D’Amico E et al (2020) Clinical and patient determinants of changing therapy in relapsing–remitting multiple sclerosis (SWITCH study). Mult Scler Relat Disord 42:102124. https://doi.org/10.1016/j.msard.2020.102124
Seifer G, Arun T, Capela C et al (2023) Influence of physicians’ risk perception on switching treatments between high-efficacy and non-high-efficacy disease-modifying therapies in multiple sclerosis. Mult Scler Relat Disord 76:104770. https://doi.org/10.1016/j.msard.2023.104770
Saccà F, Lanzillo R, Signori A et al (2019) Determinants of therapy switch in multiple sclerosis treatment-naïve patients: a real-life study. Mult Scler 25(9):1263–1272. https://doi.org/10.1177/1352458518790390
Hillert J, Magyari M, SoelbergSørensen P et al (2021) Treatment switching and discontinuation over 20 years in the big multiple sclerosis data network. Front Neurol 12:647811. https://doi.org/10.3389/fneur.2021.647811. (Published 2021 Mar 17)
Chisari CG, Sgarlata E, Arena S, Toscano S, Luca M, Patti F (2022) Rituximab for the treatment of multiple sclerosis: a review. J Neurol 269(1):159–183. https://doi.org/10.1007/s00415-020-10362-z
Pontieri L, Blinkenberg M, Bramow S et al (2022) Ocrelizumab treatment in multiple sclerosis: a Danish population-based cohort study. Eur J Neurol 29(2):496–504. https://doi.org/10.1111/ene.15142
Lamb YN (2022) Ocrelizumab: a review in multiple sclerosis. Drugs 82(3):323–334. https://doi.org/10.1007/s40265-022-01672-9
Zhu C, Kalincik T, Horakova D et al (2023) Comparison between dimethyl fumarate, fingolimod, and ocrelizumab after natalizumab cessation. JAMA Neurol 80(7):739–748. https://doi.org/10.1001/jamaneurol.2023.1542
Iaffaldano P, Lucisano G, Caputo F et al (2021) Long-term disability trajectories in relapsing multiple sclerosis patients treated with early intensive or escalation treatment strategies. Ther Adv Neurol Disord 14:17562864211019574. https://doi.org/10.1177/17562864211019574. (Published 2021 May 31)
Kappos L (2021) No consensus about consensus? Neurol Res Pract. 3(1):46. https://doi.org/10.1186/s42466-021-00144-x. (Published 2021 Aug 6)
Filippi M, Amato MP, Centonze D et al (2022) Early use of high-efficacy disease-modifying therapies makes the difference in people with multiple sclerosis: an expert opinion [published correction appears in J Neurol. 2022 Dec;269(12):6690–6691]. J Neurol 269(10):5382–5394. https://doi.org/10.1007/s00415-022-11193-w
Butzkueven H, Kappos L, Wiendl H et al (2020) Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP). J Neurol Neurosurg Psychiatry 91(6):660–668. https://doi.org/10.1136/jnnp-2019-322326
Jakimovski D, Vaughn CB, Eckert S, Zivadinov R, Weinstock-Guttman B (2020) Long-term drug treatment in multiple sclerosis: safety success and concerns. Expert Opin Drug Saf 19(9):1121–1142. https://doi.org/10.1080/14740338.2020.1805430
Funding
Novartis Pharmaceuticals Corporation, This project was supported by Novartis Italy on a basis of a sponsored research agreement between Novartis, the University of Bari Aldo Moro.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no conflicts of interest with respect to the contents of the current study, but note that the patients in the study were treated with a number of disease-modifying drugs and that authors have received advisory board, membership, speakers honoraria, travel support, research grants, consulting fees, or clinical trial support from the manufacturers of those drugs, including Actelion, Allergan, Almirall, Alexion, Bayer Schering, Biogen, Celgene, Excemed, Genzyme, Forward Pharma, Ipsen, Medday, Merck, Merz, Mylan, Novartis, Sanofi, Roche, Teva, and their local affiliates.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iaffaldano, P., Lucisano, G., Guerra, T. et al. Evaluation of drivers of treatment switch in relapsing multiple sclerosis: a study from the Italian MS Registry. J Neurol 271, 1150–1159 (2024). https://doi.org/10.1007/s00415-023-12137-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-12137-8