Abstract
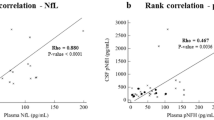
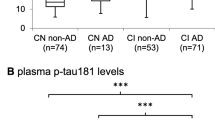
Plasma neurofilament light chain (NfL) is a promising biomarker of axonal damage for the diagnosis of neurodegenerative diseases. Phosphorylated neurofilament heavy chain (pNfH) has demonstrated its value in motor neuron diseases diagnosis, but has less been explored for dementia diagnosis. In a cross-sectional study, we compared cerebrospinal fluid (CSF) and plasma NfL and pNfH levels in n = 188 patients from Lariboisière Hospital, Paris, France, including AD patients at mild cognitive impairment stage (AD-MCI, n = 36) and dementia stage (n = 64), non-AD MCI (n = 38), non-AD dementia (n = 28) patients and control subjects (n = 22). Plasma NfL, plasma and CSF pNfH levels were measured using Simoa and CSF NfL using ELISA. The correlation between CSF and plasma levels was stronger for NfL than pNfH (rho = 0.77 and rho = 0.52, respectively). All neurofilament markers were increased in AD-MCI, AD dementia and non-AD dementia groups compared with controls. CSF NfL, CSF pNfH and plasma NfL showed high performance to discriminate AD at both MCI and dementia stages from control subjects [AUC (area under the curve) = 0.82–0.91]. Plasma pNfH displayed overall lower AUCs for discrimination between groups compared with CSF pNfH. Neurofilament markers showed similar moderate association with cognition. NfL levels displayed significant association with mediotemporal lobe atrophy and white matter lesions in the AD group. Our results suggest that CSF NfL and pNfH as well as plasma NfL levels display equivalent performance in both positive and differential AD diagnosis in memory clinic settings. In contrast to motoneuron disorders, plasma pNfH did not demonstrate added value as compared with plasma NfL.





Similar content being viewed by others
Data availability
Anonymized data can be shared by request from qualified investigators; providing data transfer is in agreement with EU legislation and decisions by the institutional review board of Lariboisière Hospital, APHP, Paris, France.
Abbreviations
- AD:
-
Alzheimer’s disease
- Aβ:
-
Amyloid beta
- ALS:
-
Amyotrophic lateral sclerosis
- AUC:
-
Area under the curve
- CSF:
-
Cerebrospinal fluid
- FTD:
-
Frontotemporal dementia
- DLB:
-
Dementia with Lewy bodies
- MMSE:
-
Mini-mental state examination
- NfL:
-
Neurofilament light chain
- NC:
-
Neurological controls
- Nf:
-
Neurofilament
- MCI:
-
Mild cognitive impairment
- MRI:
-
Magnetic resonance imaging
- PET:
-
Positron emission tomography
- pNfH:
-
Phosphorylated neurofilament heavy chain
- ROC:
-
Receiver operator characteristic
- Simoa:
-
Single molecule array
- VaD:
-
Vascular dementia
References
Bomont P (2021) The dazzling rise of neurofilaments: physiological functions and roles as biomarkers. Curr Opin Cell Biol 68:181–191. https://doi.org/10.1016/j.ceb.2020.10.011
Yuan A, Veeranna MVR, Nixon RA (2017) Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol 9:a018309. https://doi.org/10.1101/cshperspect.a018309
Ashton NJ, Leuzy A, Lim YM, Troakes C, Hortobágyi T, Höglund K et al (2019) Increased plasma neurofilament light chain concentration correlates with severity of post-mortem neurofibrillary tangle pathology and neurodegeneration. Acta Neuropathol Commun 7:5. https://doi.org/10.1186/s40478-018-0649-3
Ashton NJ, Janelidze S, Al Khleifat A, Leuzy A, van der Ende EL, Karikari TK et al (2021) A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat Commun 12:3400. https://doi.org/10.1038/s41467-021-23620-z
Simrén J, Andreasson U, Gobom J, Suarez Calvet M, Borroni B, Gillberg C et al (2022) Establishment of reference values for plasma neurofilament light based on healthy individuals aged 5–90 years. Brain Commun 4:fcac174. https://doi.org/10.1093/braincomms/fcac174
Benedet AL, Leuzy A, Pascoal TA, Ashton NJ, Mathotaarachchi S, Savard M et al (2020) Stage-specific links between plasma neurofilament light and imaging biomarkers of Alzheimer’s disease. Brain 143:3793–3804. https://doi.org/10.1093/brain/awaa342
Steinacker P, Feneberg E, Weishaupt J, Brettschneider J, Tumani H, Andersen PM et al (2016) Neurofilaments in the diagnosis of motoneuron diseases: a prospective study on 455 patients. J Neurol Neurosurg Psychiatry 87:12–20. https://doi.org/10.1136/jnnp-2015-311387
De Schaepdryver M, Goossens J, De Meyer S, Jeromin A, Masrori P, Brix B et al (2019) Serum neurofilament heavy chains as early marker of motor neuron degeneration. Ann Clin Transl Neurol 6:1971–1979. https://doi.org/10.1002/acn3.50890
Poesen K, Van Damme P (2019) Diagnostic and prognostic performance of neurofilaments in ALS. Front Neurol 9:1167. https://doi.org/10.3389/fneur.2018.01167
Feneberg E, Oeckl P, Steinacker P, Verde F, Barro C, Van Damme P et al (2018) Multicenter evaluation of neurofilaments in early symptom onset amyotrophic lateral sclerosis. Neurology 90:e22-30. https://doi.org/10.1212/WNL.0000000000004761
Bridel C, van Wieringen WN, Zetterberg H, Tijms BM, Teunissen CE, the NFL Group (2019) Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol 76:1035–1048. https://doi.org/10.1001/jamaneurol.2019.1534
Abu-Rumeileh S, Abdelhak A, Foschi M, D’Anna L, Russo M, Steinacker P et al (2023) The multifaceted role of neurofilament light chain protein in non-primary neurological diseases. Brain 146:421–437. https://doi.org/10.1093/brain/awac328
Rohrer JD, Woollacott IOC, Dick KM, Brotherhood E, Gordon E, Fellows A et al (2016) Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology 87:1329–1336. https://doi.org/10.1212/WNL.0000000000003154
Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K (2019) Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol 76:791–799. https://doi.org/10.1001/jamaneurol.2019.0765
Hayer SN, Liepelt I, Barro C, Wilke C, Kuhle J, Martus P et al (2020) NfL and pNfH are increased in Friedreich’s ataxia. J Neurol 267:1420–1430. https://doi.org/10.1007/s00415-020-09722-6
Peng L, Wan L, Liu M, Long Z, Chen D, Yuan X et al (2023) Diagnostic and prognostic performance of plasma neurofilament light chain in multiple system atrophy: a cross-sectional and longitudinal study. J Neurol. https://doi.org/10.1007/s00415-023-11741-y
Lin Y-S, Lee W-J, Wang S-J, Fuh J-L (2018) Levels of plasma neurofilament light chain and cognitive function in patients with Alzheimer or Parkinson disease. Sci Rep 8:17368. https://doi.org/10.1038/s41598-018-35766-w
Preische O, Schultz SA, Apel A, Kuhle J, Kaeser SA, Barro C et al (2019) Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer’s disease. Nat Med 25:277–283. https://doi.org/10.1038/s41591-018-0304-3
Eratne D, Loi SM, Li Q-X, Stehmann C, Malpas CB, Santillo A et al (2022) Cerebrospinal fluid neurofilament light chain differentiates primary psychiatric disorders from rapidly progressive, Alzheimer’s disease and frontotemporal disorders in clinical settings. Alzheimers Dement. https://doi.org/10.1002/alz.12549
Katisko K, Cajanus A, Jääskeläinen O, Kontkanen A, Hartikainen P, Korhonen VE et al (2020) Serum neurofilament light chain is a discriminative biomarker between frontotemporal lobar degeneration and primary psychiatric disorders. J Neurol 267:162–167. https://doi.org/10.1007/s00415-019-09567-8
Sarto J, Ruiz-García R, Guillén N, Ramos-Campoy Ó, Falgàs N, Esteller D et al (2023) Diagnostic performance and clinical applicability of blood-based biomarkers in a prospective memory clinic cohort. Neurology 100:e860–e873. https://doi.org/10.1212/WNL.0000000000201597
Ferreira PCL, Zhang Y, Snitz B, Chang CCH, Bellaver B, Jacobsen E et al (2023) Plasma biomarkers identify older adults at risk of Alzheimer’s disease and related dementias in a real-world population-based cohort. Alzheimers Dement. https://doi.org/10.1002/alz.12986
Götze K, Vrillon A, Bouaziz-Amar E, Mouton-Liger F, Hugon J, Martinet M et al (2023) Plasma neurofilament light chain in memory clinic practice: Evidence from a real-life study. Neurobiol Dis 176:105937. https://doi.org/10.1016/j.nbd.2022.105937
Eratne D, Keem M, Lewis C, Kang M, Walterfang M, Loi S et al (2022) Cerebrospinal fluid neurofilament light chain differentiates behavioural variant frontotemporal dementia progressors from ‘phenocopy’ non-progressors. Neurology. https://doi.org/10.1101/2022.01.14.22269323
Wilke C, Pujol-Calderón F, Barro C, Stransky E, Blennow K, Michalak Z et al (2019) Correlations between serum and CSF pNfH levels in ALS, FTD and controls: a comparison of three analytical approaches. Clin Chem Lab Med 57:1556–1564. https://doi.org/10.1515/cclm-2019-0015
Escal J, Fourier A, Formaglio M, Zimmer L, Bernard E, Mollion H et al (2021) Comparative diagnosis interest of NfL and pNfH in CSF and plasma in a context of FTD–ALS spectrum. J Neurol. https://doi.org/10.1007/s00415-021-10714-3
Benatar M, Zhang L, Wang L, Granit V, Statland J, Barohn R et al (2020) Validation of serum neurofilaments as prognostic and potential pharmacodynamic biomarkers for ALS. Neurology 95:e59-69. https://doi.org/10.1212/WNL.0000000000009559
Gendron TF, Daughrity LM, Heckman MG, Diehl NN, Wuu J, C9ORF72 Neurofilament Study Group et al (2017) Phosphorylated neurofilament heavy chain: a biomarker of survival for C9ORF72-associated amyotrophic lateral sclerosis. Ann Neurol 82:139–146. https://doi.org/10.1002/ana.24980
Benatar M, Wuu J, Lombardi V, Jeromin A, Bowser R, Andersen PM et al (2019) Neurofilaments in pre-symptomatic ALS and the impact of genotype. Amyotroph Lateral Scler Frontotemporal Degener 20:538–548. https://doi.org/10.1080/21678421.2019.1646769
Baumgartner D, Mazanec R, Hanzalová J (2023) Diagnostic utility of neurofilament markers for MND is limited in restricted disease phenotype and for differentiation from compressive myeloradiculopathies. J Neurol 270:1600–1614. https://doi.org/10.1007/s00415-022-11504-1
Rossi G, Gasparoli E, Pasquali C, Di Fede G, Testa D, Albanese A et al (2004) Progressive supranuclear palsy and Parkinson’s disease in a family with a new mutation in the tau gene. Ann Neurol 55:448. https://doi.org/10.1002/ana.20006
Falzone YM, Domi T, Agosta F, Pozzi L, Schito P, Fazio R et al (2020) Serum phosphorylated neurofilament heavy-chain levels reflect phenotypic heterogeneity and are an independent predictor of survival in motor neuron disease. J Neurol 267:2272–2280. https://doi.org/10.1007/s00415-020-09838-9
de Jong D, Jansen RWMM, Pijnenburg YAL, van Geel WJA, Borm GF, Kremer HPH et al (2007) CSF neurofilament proteins in the differential diagnosis of dementia. J Neurol Neurosurg Psychiatry 78:936–938. https://doi.org/10.1136/jnnp.2006.107326
Behzadi A, Pujol-Calderón F, Tjust AE, Wuolikainen A, Höglund K, Forsberg K et al (2021) Neurofilaments can differentiate ALS subgroups and ALS from common diagnostic mimics. Sci Rep 11:22128. https://doi.org/10.1038/s41598-021-01499-6
Poesen K, De Schaepdryver M, Stubendorff B, Gille B, Muckova P, Wendler S et al (2017) Neurofilament markers for ALS correlate with extent of upper and lower motor neuron disease. Neurology 88:2302–2309. https://doi.org/10.1212/WNL.0000000000004029
Halbgebauer S, Steinacker P, Verde F, Weishaupt J, Oeckl P, von Arnim C et al (2021) Comparison of CSF and serum neurofilament light and heavy chain as differential diagnostic biomarkers for ALS. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp-2021-327129
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC et al (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:270–279. https://doi.org/10.1016/j.jalz.2011.03.008
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:263–269. https://doi.org/10.1016/j.jalz.2011.03.005
Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB et al (2018) NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14:535–562. https://doi.org/10.1016/j.jalz.2018.02.018
Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF et al (2011) Classification of primary progressive aphasia and its variants. Neurology 76:1006–1014. https://doi.org/10.1212/WNL.0b013e31821103e6
Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J et al (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134:2456–2477. https://doi.org/10.1093/brain/awr179
McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor J-P, Weintraub D et al (2017) Diagnosis and management of dementia with Lewy bodies. Neurology 89:88–100. https://doi.org/10.1212/WNL.0000000000004058
Sachdev P, Kalaria R, O’Brien J, Skoog I, Alladi S, Black SE et al (2014) Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord 28:206–218. https://doi.org/10.1097/WAD.0000000000000034
Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P et al (1992) Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry 55:967–972. https://doi.org/10.1136/jnnp.55.10.967
Leitão MJ, Silva-Spínola A, Santana I, Olmedo V, Nadal A, Le Bastard N et al (2019) Clinical validation of the Lumipulse G cerebrospinal fluid assays for routine diagnosis of Alzheimer’s disease. Alzheimers Res Ther 11:91. https://doi.org/10.1186/s13195-019-0550-8
Rissin DM, Kan CW, Campbell TG, Howes SC, Fournier DR, Song L et al (2010) Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol 28:595–599. https://doi.org/10.1038/nbt.1641
Gaetani L, Höglund K, Parnetti L, Pujol-Calderon F, Becker B, Eusebi P et al (2018) A new enzyme-linked immunosorbent assay for neurofilament light in cerebrospinal fluid: analytical validation and clinical evaluation. Alzheimers Res Ther. https://doi.org/10.1186/s13195-018-0339-1
Dumurgier J, Laplanche J-L, Mouton-Liger F, Lapalus P, Indart S, Prévot M et al (2014) The screening of Alzheimer’s patients with CSF biomarkers, modulates the distribution of APOE genotype: impact on clinical trials. J Neurol 261:1187–1195. https://doi.org/10.1007/s00415-014-7335-6
Eid M, Gollwitzer M, Schmitt M (2010) Statistik und Forschungsmethoden. Technische Universität Dortmund. https://doi.org/10.17877/DE290R-12739
Jacqmin-Gadda H, Fabrigoule C, Commenges D, Dartigues JF (1997) A 5-year longitudinal study of the mini-mental state examination in normal aging. Am J Epidemiol 145:498–506. https://doi.org/10.1093/oxfordjournals.aje.a009137
Li S, Ren Y, Zhu W, Yang F, Zhang X, Huang X (2016) Phosphorylated neurofilament heavy chain levels in paired plasma and CSF of amyotrophic lateral sclerosis. J Neurol Sci 367:269–274. https://doi.org/10.1016/j.jns.2016.05.062
Alagaratnam J, von Widekind S, De Francesco D, Underwood J, Edison P, Winston A et al (2021) Correlation between CSF and blood neurofilament light chain protein: a systematic review and meta-analysis. BMJ Neurol Open 3:e000143. https://doi.org/10.1136/bmjno-2021-000143
Kushkuley J, Metkar S, Chan WK-H, Lee S, Shea TB (2010) Aluminum induces neurofilament aggregation by stabilizing cross-bridging of phosphorylated c-terminal sidearms. Brain Res 1322:118–123. https://doi.org/10.1016/j.brainres.2010.01.075
Lu C-H, Kalmar B, Malaspina A, Greensmith L, Petzold A (2011) A method to solubilise protein aggregates for immunoassay quantification which overcomes the neurofilament “hook” effect. J Neurosci Methods 195:143–150. https://doi.org/10.1016/j.jneumeth.2010.11.026
Khalil M, Pirpamer L, Hofer E, Voortman MM, Barro C, Leppert D et al (2020) Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun 11:812. https://doi.org/10.1038/s41467-020-14612-6
Steinacker P, Anderl-Straub S, Diehl-Schmid J, Semler E, Uttner I, von Arnim CAF et al (2018) Serum neurofilament light chain in behavioral variant frontotemporal dementia. Neurology 91:e1390–e1401. https://doi.org/10.1212/WNL.0000000000006318
Giacomucci G, Mazzeo S, Bagnoli S, Ingannato A, Leccese D, Berti V et al (2022) Plasma neurofilament light chain as a biomarker of Alzheimer’s disease in subjective cognitive decline and mild cognitive impairment. J Neurol 269:4270–4280. https://doi.org/10.1007/s00415-022-11055-5
De Schaepdryver M, Jeromin A, Gille B, Claeys KG, Herbst V, Brix B et al (2018) Comparison of elevated phosphorylated neurofilament heavy chains in serum and cerebrospinal fluid of patients with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 89:367–373. https://doi.org/10.1136/jnnp-2017-316605
Oeckl P, Jardel C, Salachas F, Lamari F, Andersen PM, Bowser R et al (2016) Multicenter validation of CSF neurofilaments as diagnostic biomarkers for ALS. Amyotroph Lateral Scler Frontotemporal Degener 17:404–413. https://doi.org/10.3109/21678421.2016.1167913
Julien JP (2001) Amyotrophic lateral sclerosis. Unfolding the toxicity of the misfolded. Cell 104:581–591. https://doi.org/10.1016/s0092-8674(01)00244-6
Meier J, Couillard-Després S, Jacomy H, Gravel C, Julien JP (1999) Extra neurofilament NF-L subunits rescue motor neuron disease caused by overexpression of the human NF-H gene in mice. J Neuropathol Exp Neurol 58:1099–1110
Hirano A, Nakano I, Kurland LT, Mulder DW, Holley PW, Saccomanno G (1984) Fine structural study of neurofibrillary changes in a family with amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 43:471–480. https://doi.org/10.1097/00005072-198409000-00002
Mielke MM, Syrjanen JA, Blennow K, Zetterberg H, Vemuri P, Skoog I et al (2019) Plasma and CSF neurofilament light: relation to longitudinal neuroimaging and cognitive measures. Neurology 93:e252–e260. https://doi.org/10.1212/WNL.0000000000007767
Ebenau JL, Pelkmans W, Verberk IMW, Verfaillie SCJ, van den Bosch KA, van Leeuwenstijn M et al (2022) Association of CSF, plasma, and imaging markers of neurodegeneration with clinical progression in people with subjective cognitive decline. Neurology 98:e1315–e1326. https://doi.org/10.1212/WNL.0000000000200035
Mattsson N, Andreasson U, Zetterberg H, Blennow K (2017) Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol 74:557–566. https://doi.org/10.1001/jamaneurol.2016.6117
de Flon P, Laurell K, Sundström P, Blennow K, Söderström L, Zetterberg H et al (2019) Comparison of plasma and cerebrospinal fluid neurofilament light in a multiple sclerosis trial. Acta Neurol Scand 139:462–468. https://doi.org/10.1111/ane.13078
Pafiti A, Krashias G, Tzartos J, Tzartos S, Stergiou C, Gaglia E et al (2023) A comparison of two analytical approaches for the quantification of neurofilament light chain, a biomarker of axonal damage in multiple sclerosis. Int J Mol Sci 24:10787. https://doi.org/10.3390/ijms241310787
Acknowledgements
The authors wish to sincerely thank all the patients who participated in this study and their relatives.
Funding
AV is funded by Fondation Ophtalmologique Adolphe de Rothschild, Fondation Philipe Chatrier, Amicale des Anciens Internes des Hôpitaux de Paris, Fondation Vaincre Alzheimer, the Swedish Dementia Foundation (Demensfonden), Gun and Bertil Stohnes Foundation and Gamla Tjänarinnor Foundation. NJA is funded by the Wallenberg Centre for Molecular and Translational Medicine, Swedish Alzheimer Foundation (Alzheimerfonden), the Swedish Dementia Foundation (Demensfonden), Hjärnfonden (#FO2020-0241) and Gamla Tjänarinnor. TKK was funded by the BrightFocus Foundation (#A2020812F), the International Society for Neurochemistry’s Career Development Grant, the Swedish Alzheimer Foundation (Alzheimerfonden; #AF-940244), the Swedish Brain Foundation (Hjärnfonden; #FO2021-0298), the Swedish Dementia Foundation (Demensförbundet), the Swedish Parkinson Foundation (Parkinsonfonden), Gamla Tjänarinnor Foundation, the Aina (Ann) Wallströms and Mary-Ann Sjöbloms Foundation, the Agneta Prytz-Folkes & Gösta Folkes Foundation (#2020-00124), the Gun and Bertil Stohnes Foundation, and the Anna Lisa and Brother Björnsson’s Foundation. HZ is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018-02532), the European Research Council (#681712), Swedish State Support for Clinical Research (#ALFGBG-720931), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer’s Association (#ADSF-21-831376-C, #ADSF-21-831381-C and #ADSF-21-831377-C), the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2019-0228), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), and the UK Dementia Research Institute at UCL.
Author information
Authors and Affiliations
Contributions
AV: acquisition of data, drafting/revision of the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data. NJA: acquisition of data, drafting/revision of the manuscript for content, study concept or design, analysis or interpretation of data. TKK: drafting/revision of the manuscript for content, study concept or design, analysis or interpretation of data. KG: acquisition of data, drafting/revision of the manuscript for content. JD: acquisition of data, drafting/revision of the manuscript for content. EC: acquisition of data, drafting/revision of the manuscript for content ML: acquisition of data, drafting/revision of the manuscript for content. HZ: drafting/revision of the manuscript for content, study concept or design. KB: drafting/revision of the manuscript for content, study concept or design, analysis or interpretation of data. CP: acquisition of data, drafting/revision of the manuscript for content, study concept or design, analysis or interpretation of data.
Corresponding author
Ethics declarations
Conflicts of interest
HZ has served at scientific advisory boards for Alector, Eisai, Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, Nervgen, AZTherapies and CogRx, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). CP is a member of the International Advisory Boards of Lilly; is a consultant for Fujiribio, Alzhois, Neuroimmune, Ads Neuroscience, Roche, AgenT and Gilead; and is involved as an investigator in several clinical trials for Roche, Esai, Lilly, Biogen, Astra-Zeneca, Lundbeck, and Neuroimmune. KB has served as a consultant and at advisory boards for Acumen, ALZPath, BioArctic, Biogen, Eisai, Lilly, Moleac Pte. Ltd, Novartis, Ono Pharma, Prothena, Roche Diagnostics, and Siemens Healthineers; has served at data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai and Roche Diagnostics; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Progra (outside submitted work).
Ethical approval
This research study was approved by the local ethics committee of Bichat Hospital, Paris, France (CEERB GHU Nord no.10-037).
Patient consent
Informed consent was obtained from the patients who participated in the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vrillon, A., Ashton, N.J., Karikari, T.K. et al. Comparison of CSF and plasma NfL and pNfH for Alzheimer’s disease diagnosis: a memory clinic study. J Neurol 271, 1297–1310 (2024). https://doi.org/10.1007/s00415-023-12066-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-12066-6