Abstract
Study objectives
To describe a case series of patients with prominent sleep disturbances and their polysomnography findings in six patients with dipeptidyl-peptidase-like protein-6 (DPPX) autoimmunity syndrome.
Methods
Of 13 patients with DPPX autoimmunity evaluated at Mayo Clinic, 6 were seen by Sleep Medicine with polysomnography and were assessed with blood and cerebrospinal fluid, neuroimaging, neuropsychological testing, and evaluation tailored to clinical presentation.
Results
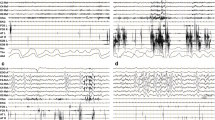
Median age of our six DPPX autoimmunity patients was 57 (range 27–70) years, with one woman. All patients had prominent gastrointestinal disturbances, most with prominent and early weight loss, and a spectrum of neuropsychiatric disturbances including cognitive impairment, myoclonus, exaggerated startle, and dysautonomia. Sleep disturbances included insomnia and obstructive sleep apnea in six patients, periodic leg movements of sleep in four, and REM sleep behavior disorder in two. Polysomnography demonstrated REM sleep-atonia loss in four patients, and ambiguous sleep with status dissociatus (mixed features of wakefulness, non-rapid eye movement [NREM], and REM sleep) appeared in one patient. Five of six patients showed neurological improvement with immunotherapy, including three with at least partial improvement in sleep disturbances.
Conclusion
Our patients with DPPX autoimmunity syndrome had prominent sleep disturbances including sleep-disordered breathing, REM sleep behavior disorder, and abnormal NREM sleep architecture with highly variable clinical presentations. DPPX autoimmunity should be considered in cases with a triad of sleep disturbance, neurological features of hyperexcitability, and systemic symptoms of gastrointestinal disturbance and weight loss. Future prospective studies of DPPX autoimmunity syndrome including detailed sleep evaluation and follow-up are necessary.

Similar content being viewed by others
Data availability
Data will be provided for qualified investigators by request to the corresponding author (EKStL).
References
Boronat A, Gelfand JM, Gresa-Arribas N et al (2013) Encephalitis and antibodies to dipeptidyl-peptidase-like protein-6, a subunit of Kv4.2 potassium channels. Ann Neurol 73:120–128. https://doi.org/10.1002/ana.23756
Tobin WO, Lennon VA, Komorowski L et al (2014) DPPX potassium channel antibody: frequency, clinical accompaniments, and outcomes in 20 patients. Neurology 83:1797–1803. https://doi.org/10.1212/WNL.0000000000000991
Hara M, Arino H, Petit-Pedrol M et al (2017) DPPX antibody-associated encephalitis: main syndrome and antibody effects. Neurology 88:1340–1348. https://doi.org/10.1212/wnl.0000000000003796
Silber MH (2016) Autoimmune sleep disorders. Handb Clin Neurol 133:317–326. https://doi.org/10.1016/B978-0-444-63432-0.00018-9
Devine MF, St Louis EK (2021) Sleep disturbances associated with neurological autoimmunity. Neurotherapeutics 18:181–201. https://doi.org/10.1007/s13311-021-01020-x
Overeem S, Dalmau J, Bataller L et al (2004) Hypocretin-1 CSF levels in anti-Ma2 associated encephalitis. Neurology 62:138–140. https://doi.org/10.1212/01.wnl.0000101718.92619.67
Rosenfeld MR, Eichen JG, Wade DF, Posner JB, Dalmau J (2001) Molecular and clinical diversity in paraneoplastic immunity to Ma proteins. Ann Neurol 50:339–348
Pittock SJ, Parisi JE, McKeon A et al (2010) Paraneoplastic jaw dystonia and laryngospasm with antineuronal nuclear autoantibody type 2 (anti-Ri). Arch Neurol 67:1109–1115. https://doi.org/10.1001/archneurol.2010.209
Gaig C, Graus F, Compta Y et al (2017) Clinical manifestations of the anti-IgLON5 disease. Neurology 88:1736–1743. https://doi.org/10.1212/wnl.0000000000003887
Hogl B, Heidbreder A, Santamaria J, Graus F, Poewe W (2015) IgLON5 autoimmunity and abnormal behaviours during sleep. Lancet 385:1590. https://doi.org/10.1016/s0140-6736(15)60445-7
Sabater L, Gaig C, Gelpi E et al (2014) A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol 13:575–586. https://doi.org/10.1016/S1474-4422(14)70051-1
Devine MF, Feemster JC, Lieske EA et al (2022) Objective sleep profile in LGI1/CASPR2 autoimmunity. Sleep. https://doi.org/10.1093/sleep/zsab297
Lin N, Hao H, Guan H et al (2020) Sleep disorders in leucine-rich glioma-inactivated protein 1 and contactin protein-like 2 antibody-associated diseases. Front Neurol 11:696. https://doi.org/10.3389/fneur.2020.00696
Liu X, Yang L, Han Y et al (2021) Electrophysiological evaluation in identifying unique sleep features among anti-LGI1 encephalitis patients during active and recovery phase. Nat Sci Sleep 13:527–536. https://doi.org/10.2147/NSS.S299467
Kokmen E, Naessens JM, Offord KP (1987) A short test of mental status: description and preliminary results. Mayo Clin Proc 62:281–288. https://doi.org/10.1016/s0025-6196(12)61905-3
Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC (1991) The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol 48:725–728. https://doi.org/10.1001/archneur.1991.00530190071018
International Classification of Sleep Disorders. American Academy of Sleep Medicine, Darien 2014
Gadoth A, Kryzer TJ, Fryer J, McKeon A, Lennon VA, Pittock SJ (2017) Microtubule-associated protein 1B: novel paraneoplastic biomarker. Ann Neurol 81:266–277. https://doi.org/10.1002/ana.24872
McCarter SJ, St. Louis EK, Duwell EJ et al (2014) Diagnostic thresholds for quantitative REM sleep phasic burst duration, phasic and tonic muscle activity, and REM Atonia index in REM sleep behavior disorder with and without comorbid obstructive sleep apnea. Sleep 37:1649–1662
McCarter SJ, St Louis EK, Sandness DJ et al (2017) Diagnostic REM sleep muscle activity thresholds in patients with idiopathic REM sleep behavior disorder with and without obstructive sleep apnea. Sleep Med 33:23–29. https://doi.org/10.1016/j.sleep.2016.03.013
Cesari M, Heidbreder A, St Louis EK et al (2021) Video-polysomnography procedures for diagnosis of rapid eye movement sleep behavior disorder (RBD) and the identification of its prodromal stages: guidelines from the International RBD Study Group. Sleep 25:25. https://doi.org/10.1093/sleep/zsab257
Iber C, Ancoli-Israel S, Chesson A, Quan SF (2007) The AASM Manual for the scoring of sleep and associated events: rules. Terminology and technical specifications, 1st edn. American Academy of Sleep Medicine, Westchester
Frauscher B, Iranzo A, Gaig C et al (2012) Normative EMG values during REM sleep for the diagnosis of REM sleep behavior disorder. Sleep 35:835–847. https://doi.org/10.5665/sleep.1886
Frauscher B, Iranzo A, Hogl B et al (2008) Quantification of electromyographic activity during REM sleep in multiple muscles in REM sleep behavior disorder. Sleep 31:724–731
Iranzo A, Frauscher B, Santos H et al (2011) Usefulness of the SINBAR electromyographic montage to detect the motor and vocal manifestations occurring in REM sleep behavior disorder. Sleep Med 12:284–288. https://doi.org/10.1016/j.sleep.2010.04.021
Iranzo A, Ratti PL, Casanova-Molla J, Serradell M, Vilaseca I, Santamaria J (2009) Excessive muscle activity increases over time in idiopathic REM sleep behavior disorder. Sleep 32:1149–1153
Piepgras J, Holtje M, Michel K et al (2015) Anti-DPPX encephalitis: pathogenic effects of antibodies on gut and brain neurons. Neurology 85:890–897. https://doi.org/10.1212/wnl.0000000000001907
Iranzo A, Aparicio J (2009) A lesson from anatomy: focal brain lesions causing REM sleep behavior disorder. Sleep Med 10:9–12. https://doi.org/10.1016/j.sleep.2008.03.005
McCarter SJ, Tippmann-Peikert M, Sandness DJ et al (2015) Neuroimaging-evident lesional pathology associated with REM sleep behavior disorder. Sleep Med 16:1502–1510. https://doi.org/10.1016/j.sleep.2015.07.018
McCarter SJ, St Louis EK, Sandness DJ et al (2015) Antidepressants increase REM sleep muscle tone in patients with and without REM sleep behavior disorder. Sleep 38:907–917. https://doi.org/10.5665/sleep.4738
McCarter SJ, Feemster JC, Tabatabai GM et al (2019) Submentalis rapid eye movement sleep muscle activity: a potential biomarker for synucleinopathy. Ann Neurol 86:969–974. https://doi.org/10.1002/ana.25622
McCarter SJ, Tabatabai GM, Jong HY et al (2020) REM sleep atonia loss distinguishes synucleinopathy in older adults with cognitive impairment. Neurology 94:e15–e29. https://doi.org/10.1212/WNL.0000000000008694
Acknowledgements
The authors also thank Ms. Lea Dacy, Department of Neurology, for secretarial support during the submission process.
Funding
This work was supported in part by the National Center for Advancing Translational Sciences grant Number UL1 TR002377, and by the National Institute on Aging grant R34 AG056639. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The following authors have nothing to disclose: Gadoth, McCarter, Gossard, Cattaneo, Devine. Non-financial Interests: S.J. Pittock has provided consultation to Alexion Pharmaceuticals and MedImmune but has received no personal fees or personal compensation for these consulting activities. A. McKeon has consulted for MedImmune, LLC, Grifols and Euroimmun but has not received personal compensation for these activities. E. St. Louis has consulted for Axovant, Inc., but has not received personal compensation for these activities. Financial Interests: S. J. Pittock has received research support from Alexion Pharmaceuticals, MedImmune and Grifols. W. O. Tobin receives research support from the National Institute of Neurological Disorders and Stroke, the National Institute of Allergy and Infectious Diseases, Alexion Pharmaceuticals, Sanofi US Services Inc., and UCB Biosciences. A. McKeon receives research support from MedImmune, LLC. E. St. Louis receives research support from the National Center for Advancing Translational Sciences, the National Institute of Neurological Disorders and Stroke, the National Heart, Lung and Blood Institute, Axovant, Inc., and Sunovion, Inc.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gadoth, A., Devine, M.F., Pittock, S.J. et al. Sleep disturbances associated with DPPX autoantibodies: a case series. J Neurol 270, 3543–3552 (2023). https://doi.org/10.1007/s00415-023-11698-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11698-y