Abstract
Objective
To describe the clinical associations of SOX1 antibodies (SOX1-Abs), determine the accuracy of various detection techniques, and propose laboratory criteria to identify definite paraneoplastic neurological syndromes (PNS) associated with SOX1-Abs.
Methods
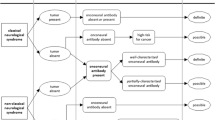
Single-center, retrospective study of patients referred to the French Reference Center between 2009 and 2019 for confirmation of SOX1-Ab positivity, without concurrent neural antibodies. Patients were classified according to the updated diagnostic PNS criteria; biological samples were systematically retested with three distinct techniques (line blot, cell-based assay, indirect immunofluorescence).
Results
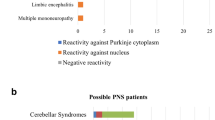
Among 77 patients with isolated SOX1-Ab positivity, 23 (29.9%) fulfilled the criteria for definite PNS; all of them had lung cancer (mostly small-cell) and presented mainly with Lambert-Eaton myasthenic syndrome (10/23) and rapidly progressive cerebellar ataxia (6/23). SOX1-Ab positivity varied depending on the laboratory methods which were used, and a single technique was not sufficient to draw conclusions about the PNS diagnosis. The combination of an antigen-specific test (line blot and/or cell-based assay) and immunofluorescence showed the highest accuracy (81.5%, 95% CI 70.0–90.1) in identifying definite PNS. Moreover, when the PNS-Care score was recalculated assigning three points at the laboratory-level only to patients with positive “antigenic-specific test + immunofluorescence” and 0 points to the remaining cases, a higher certainty for definite and non-PNS was achieved (from 41/77, 53.2%, to 60/77, 77.9%; p < 0.001).
Conclusion
SOX1-Abs should be considered high-risk antibodies only when detected with a positive antigenic-specific test and immunofluorescence. Other laboratory results and clinical associations different from Lambert-Eaton myasthenic syndrome and rapidly progressive cerebellar ataxia should be carefully reassessed to rule out false positivity and alternative diagnoses.


Similar content being viewed by others
Data availability
Any data not published within the article are available and will be shared by request from any qualified investigator.
References
Vural B, Chen L-C, Saip P et al (2005) Frequency of SOX Group B (SOX1, 2, 3) and ZIC2 antibodies in Turkish patients with small cell lung carcinoma and their correlation with clinical parameters. Cancer 103:2575–2583. https://doi.org/10.1002/cncr.21088
Güre AO, Stockert E, Scanlan MJ et al (2000) Serological identification of embryonic neural proteins as highly immunogenic tumor antigens in small cell lung cancer. Proc Natl Acad Sci USA 97:4198–4203. https://doi.org/10.1073/pnas.97.8.4198
Graus F, Vincent A, Pozo-Rosich P et al (2005) Anti-glial nuclear antibody: marker of lung cancer-related paraneoplastic neurological syndromes. J Neuroimmunol 165:166–171. https://doi.org/10.1016/j.jneuroim.2005.03.020
Sabater L, Titulaer M, Saiz A et al (2008) SOX1 antibodies are markers of paraneoplastic Lambert-Eaton myasthenic syndrome. Neurology 70:924–928. https://doi.org/10.1212/01.wnl.0000281663.81079.24
Titulaer MJ, Klooster R, Potman M et al (2009) SOX antibodies in small-cell lung cancer and lambert-eaton myasthenic syndrome: frequency and relation with survival. J Clin Oncol 27:4260–4267. https://doi.org/10.1200/JCO.2008.20.6169
Sabater L, Höftberger R, Boronat A et al (2013) Antibody repertoire in paraneoplastic cerebellar degeneration and small cell lung cancer. PLoS ONE 8:e60438. https://doi.org/10.1371/journal.pone.0060438
Tschernatsch M, Singh P, Gross O et al (2010) Anti-SOX1 antibodies in patients with paraneoplastic and non-paraneoplastic neuropathy. J Neuroimmunol 226:177–180. https://doi.org/10.1016/j.jneuroim.2010.07.005
Berger B, Dersch R, Ruthardt E et al (2016) Prevalence of anti-SOX1 reactivity in various neurological disorders. J Neurol Sci 369:342–346. https://doi.org/10.1016/j.jns.2016.09.002
Maddison P, Titulaer MJ, Verschuuren JJ et al (2019) The utility of anti-SOX2 antibodies for cancer prediction in patients with paraneoplastic neurological disorders. J Neuroimmunol 326:14–18. https://doi.org/10.1016/j.jneuroim.2018.11.003
Déchelotte B, Muñiz-Castrillo S, Joubert B et al (2020) Diagnostic yield of commercial immunodots to diagnose paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm 7:e701. https://doi.org/10.1212/NXI.0000000000000701
Graus F, Vogrig A, Muñiz-Castrillo S et al (2021) Updated diagnostic criteria for paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm. https://doi.org/10.1212/NXI.0000000000001014
Chapman CJ, Thorpe AJ, Murray A et al (2011) Immunobiomarkers in small cell lung cancer: potential early cancer signals. Clin Cancer Res 17:1474–1480. https://doi.org/10.1158/1078-0432.CCR-10-1363
Kiefer JC (2007) Back to basics: sox genes. Dev Dyn 236:2356–2366. https://doi.org/10.1002/dvdy.21218
Stich O, Klages E, Bischler P et al (2012) SOX1 antibodies in sera from patients with paraneoplastic neurological syndromes: SOX1 autoimmunity. Acta Neurol Scand 125:326–331. https://doi.org/10.1111/j.1600-0404.2011.01572.x
Ruiz-García R, Martínez-Hernández E, García-Ormaechea M et al (2019) Caveats and pitfalls of SOX1 autoantibody testing with a commercial line blot assay in paraneoplastic neurological investigations. Front Immunol 10:769. https://doi.org/10.3389/fimmu.2019.00769
Acknowledgements
We thank NeuroBioTec Hospices Civils de Lyon BRC (France, AC-2013-1867, NFS96-900) for banking sera and CSF samples.
Funding
This work is supported by a public grant overseen by the Agence nationale de la recherche (ANR; French research agency) as part of the “Investissements d’Avenir” program (ANR-18-RHUS-0012). This study was performed within the framework of the LABEX CORTEX of the Université Claude Bernard Lyon 1, within the program “Investissements d'Avenir” (ANR-11-LABX-0042) operated by the ANR.
Author information
Authors and Affiliations
Contributions
Study concept and design: MV, JH, SMC. Acquisition of data: MV, ALP, AV, DG, VR, BJ, NF, JH, SMC. Analysis and interpretation of data: MV, ALP, AV, JH, SMC. Drafting of the manuscript: MV, JH, SMC. Critical revision of the manuscript for important intellectual content: MV, ALP, AV, DG, VR, BJ, NF, JH, SMC. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
Observational studies conducted in France using data obtained from a retrospective study without any additional therapeutic or monitoring procedure do not require informed consent. Patient records and information were anonymized prior to analysis. The Institutional Review Board of the University Claude Bernard Lyon 1 and Hospices Civils de Lyon approved the study.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vabanesi, M., Pinto, AL., Vogrig, A. et al. SOX1 antibody-related paraneoplastic neurological syndromes: clinical correlates and assessment of laboratory diagnostic techniques. J Neurol 270, 1691–1701 (2023). https://doi.org/10.1007/s00415-022-11523-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11523-y