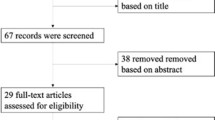
Abstract
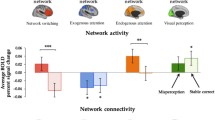
Visual hallucinations (VH) are present in up to 75% of Parkinson’s disease (PD) patients. However, their neural bases and participation of the visual system in VH are not well-understood in PD. Seventy-four participants, 12 PD with VH (PDVH), 35 PD without VH (PDnoVH) and 27 controls underwent a battery of primary visual function and visual cognition tests, retinal optical coherence tomography and structural and resting-state functional brain MRI. We quantified cortical thickness with Freesurfer and functional connectivity (FC) of Visual (VIS), Fronto-Parietal (FP), Ventral Attention (VAN) and Dorsal Attention (DAN) networks with CONN toolbox. Group comparisons were performed with MANCOVA. Area Under the Curve (AUC) was computed to assess the ability of visual variables to differentiate PDVH and PDnoVH. There were no significant PDVH vs PDnoVH differences in disease duration, motor manifestations, general cognition or dopamine agonist therapy (DA) use. Compared to PDnoVH and HC, and regardless of DA use, PDVH showed significantly reduced contrast sensitivity, visuoperceptive and visuospatial abilities, increased retina photoreceptor layer thickness, reduced cortical thickness mostly in right visual associative areas, decreased between-network VIS–VAN and VAN–DAN connectivity and increased within-network DAN connectivity. The combination of clinical and imaging variables that best discriminated PDVH and PDnoVH (highest AUC), where within-network DAN FC, photoreceptor layer thickness and cube analysis test from Visual Object and Space Perception Battery (accuracy of 81.8%). Compared to PDnoVH, PDVH have specific functional and structural abnormalities within the visual system, which can be quantified non-invasively and could potentially constitute biomarkers for VH in PD.



Similar content being viewed by others
Change history
06 December 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00415-022-11492-2
References
Diederich NJ, Fenelon G, Stebbins G, Goetz CG (2009) Hallucinations in Parkinson disease. Nat Rev Neurol 5:331–342
Ffytche DH, Creese B, Politis M, Chaudhuri KR, Weintraub D, Ballard C, Aarsland D (2017) The psychosis spectrum in Parkinson disease. Nat Rev Neurol 13:81–95
Anang JB, Gagnon JF, Bertrand JA, Romenets SR, Latreille V, Panisset M, Montplaisir J, Postuma RB (2014) Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology 83:1253–1260
Ramirez-Ruiz B, Junque C, Marti MJ, Valldeoriola F, Tolosa E (2006) Neuropsychological deficits in Parkinson’s disease patients with visual hallucinations. Mov Disord 21:1483–1487
Koerts J, Borg MA, Meppelink AM, Leenders KL, van Beilen M, van Laar T (2010) Attentional and perceptual impairments in Parkinson’s disease with visual hallucinations. Parkinsonism Relat Disord 16:270–274
Matsui H, Udaka F, Tamura A, Oda M, Kubori T, Nishinaka K, Kameyama M (2006) Impaired visual acuity as a risk factor for visual hallucinations in Parkinson’s disease. J Geriatr Psychiatry Neurol 19:36–40
Visser F, Apostolov VI, Vlaar AMM, Twisk JWR, Weinstein HC, Berendse HW (2020) Visual hallucinations in Parkinson’s disease are associated with thinning of the inner retina. Sci Rep 10:21110
Firbank MJ, Parikh J, Murphy N, Killen A, Allan CL, Collerton D, Blamire AM, Taylor JP (2018) Reduced occipital GABA in Parkinson disease with visual hallucinations. Neurology 91:e675–e685
Oishi N, Udaka F, Kameyama M, Sawamoto N, Hashikawa K, Fukuyama H (2005) Regional cerebral blood flow in Parkinson disease with nonpsychotic visual hallucinations. Neurology 65:1708–1715
Shine JM, Muller AJ, O’Callaghan C, Hornberger M, Halliday GM, Lewis SJ (2015) Abnormal connectivity between the default mode and the visual system underlies the manifestation of visual hallucinations in Parkinson’s disease: a task-based fMRI study. NPJ Parkinsons Dis 1:15003
Mosimann UP, Collerton D, Dudley R, Meyer TD, Graham G, Dean JL, Bearn D, Killen A, Dickinson L, Clarke MP, McKeith IG (2008) A semi-structured interview to assess visual hallucinations in older people. Int J Geriatr Psychiatry 23:712–718
Holiday KA, Pirogovsky-Turk E, Malcarne VL, Filoteo JV, Litvan I, Lessig SL, Song D, Schiehser DM (2017) Psychometric Properties and Characteristics of the North-East Visual Hallucinations Interview in Parkinson’s Disease. Mov Disord Clin Pract 4:717–723
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Dalrymple-Alford JC, MacAskill MR, Nakas CT, Livingston L, Graham C, Crucian GP, Melzer TR, Kirwan J, Keenan R, Wells S, Porter RJ, Watts R, Anderson TJ (2010) The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology 75:1717–1725
Tewarie P, Balk L, Costello F, Green A, Martin R, Schippling S, Petzold A (2012) The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS ONE 7:e34823
Aytulun A, Cruz-Herranz A, Aktas O, Balcer LJ, Balk L, Barboni P, Blanco AA, Calabresi PA, Costello F, Sanchez-Dalmau B, DeBuc DC, Feltgen N, Finger RP, Frederiksen JL, Frohman E, Frohman T, Garway-Heath D, Gabilondo I, Graves JS, Green AJ, Hartung HP, Havla J, Holz FG, Imitola J, Kenney R, Klistorner A, Knier B, Korn T, Kolbe S, Kramer J, Lagreze WA, Leocani L, Maier O, Martinez-Lapiscina EH, Meuth S, Outteryck O, Paul F, Petzold A, Pihl-Jensen G, Preiningerova JL, Rebolleda G, Ringelstein M, Saidha S, Schippling S, Schuman JS, Sergott RC, Toosy A, Villoslada P, Wolf S, Yeh EA, Yu-Wai-Man P, Zimmermann HG, Brandt AU, Albrecht P (2021) APOSTEL 2.0 recommendations for reporting quantitative optical coherence tomography studies. Neurology 97:68–79
Fischl B (2012) FreeSurfer. Neuroimage 62:774–781
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980
Whitfield-Gabrieli S, Nieto-Castanon A (2012) Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2:125–141
Mattay VS, Tessitore A, Callicott JH, Bertolino A, Goldberg TE, Chase TN, Hyde TM, Weinberger DR (2002) Dopaminergic modulation of cortical function in patients with Parkinson’s disease. Ann Neurol 51:156–164
Archibald NK, Clarke MP, Mosimann UP, Burn DJ (2009) The retina in Parkinson’s disease. Brain 132:1128–1145
Moller JC, Eggert KM, Unger M, Odin P, Chaudhuri KR, Oertel WH (2008) Clinical risk-benefit assessment of dopamine agonists. Eur J Neurol 15(Suppl 2):15–23
Lee JY, Kim JM, Ahn J, Kim HJ, Jeon BS, Kim TW (2014) Retinal nerve fiber layer thickness and visual hallucinations in Parkinson’s disease. Mov Disord 29:61–67
Kopal A, Mejzlikova E, Preiningerova JL, Brebera D, Ulmanova O, Ehler E, Roth J (2015) Changes of retina are not involved in the genesis of visual hallucinations in Parkinson’s disease. Parkinsons Dis 2015:709191
Marques A, Beze S, Pereira B, Chassain C, Monneyron N, Delaby L, Lambert C, Fontaine M, Derost P, Debilly B, Rieu I, Lewis SJG, Chiambaretta F, Durif F (2020) Visual hallucinations and illusions in Parkinson’s disease: the role of ocular pathology. J Neurol 267:2829–2841
Diederich NJ, Goetz CG, Raman R, Pappert EJ, Leurgans S, Piery V (1998) Poor visual discrimination and visual hallucinations in Parkinson’s disease. Clin Neuropharmacol 21:289–295
Muller AJ, O’Callaghan C, Walton CC, Shine JM, Lewis SJ (2017) Retrospective neuropsychological profile of patients with Parkinson disease prior to developing visual hallucinations. J Geriatr Psychiatry Neurol 30:90–95
Pezzoli S, Cagnin A, Busse C, Zorzi G, Fragiacomo F, Bandmann O, Venneri A (2021) Cognitive correlates and baseline predictors of future development of visual hallucinations in dementia with Lewy bodies. Cortex 142:74–83
Delli Pizzi S, Franciotti R, Tartaro A, Caulo M, Thomas A, Onofrj M, Bonanni L (2014) Structural alteration of the dorsal visual network in DLB patients with visual hallucinations: a cortical thickness MRI study. PLoS ONE 9:e86624
Bejr-Kasem H, Pagonabarraga J, Martinez-Horta S, Sampedro F, Marin-Lahoz J, Horta-Barba A, Aracil-Bolanos I, Perez-Perez J, Angeles Boti M, Campolongo A, Izquierdo C, Pascual-Sedano B, Gomez-Anson B, Kulisevsky J (2019) Disruption of the default mode network and its intrinsic functional connectivity underlies minor hallucinations in Parkinson’s disease. Mov Disord 34:78–86
Goldman JG, Stebbins GT, Dinh V, Bernard B, Merkitch D, deToledo-Morrell L, Goetz CG (2014) Visuoperceptive region atrophy independent of cognitive status in patients with Parkinson’s disease with hallucinations. Brain 137:849–859
Gama RL, Bruin VM, Tavora DG, Duran FL, Bittencourt L, Tufik S (2014) Structural brain abnormalities in patients with Parkinson’s disease with visual hallucinations: a comparative voxel-based analysis. Brain Cogn 87:97–103
Shin S, Lee JE, Hong JY, Sunwoo MK, Sohn YH, Lee PH (2012) Neuroanatomical substrates of visual hallucinations in patients with non-demented Parkinson’s disease. J Neurol Neurosurg Psychiatry 83:1155–1161
Ibarretxe-Bilbao N, Ramirez-Ruiz B, Junque C, Marti MJ, Valldeoriola F, Bargallo N, Juanes S, Tolosa E (2010) Differential progression of brain atrophy in Parkinson’s disease with and without visual hallucinations. J Neurol Neurosurg Psychiatry 81:650–657
Vossel S, Geng JJ, Fink GR (2014) Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist 20:150–159
Yau Y, Zeighami Y, Baker TE, Larcher K, Vainik U, Dadar M, Fonov VS, Hagmann P, Griffa A, Misic B, Collins DL, Dagher A (2018) Network connectivity determines cortical thinning in early Parkinson’s disease progression. Nat Commun 9:12
Espeseth T, Westlye LT, Fjell AM, Walhovd KB, Rootwelt H, Reinvang I (2008) Accelerated age-related cortical thinning in healthy carriers of apolipoprotein E epsilon 4. Neurobiol Aging 29:329–340
Fortea J, Sala-Llonch R, Bartres-Faz D, Bosch B, Llado A, Bargallo N, Molinuevo JL, Sanchez-Valle R (2010) Increased cortical thickness and caudate volume precede atrophy in PSEN1 mutation carriers. J Alzheimers Dis 22:909–922
Pagonabarraga J, Soriano-Mas C, Llebaria G, Lopez-Sola M, Pujol J, Kulisevsky J (2014) Neural correlates of minor hallucinations in non-demented patients with Parkinson’s disease. Parkinsonism Relat Disord 20:290–296
Sawczak CM, Barnett AJ, Cohn M (2019) Increased cortical thickness in attentional networks in Parkinson’s disease with minor hallucinations. Parkinsons Dis 2019:5351749
Gao Z, Zhu Q, Zhang Y, Zhao Y, Cai L, Shields CB, Cai J (2013) Reciprocal modulation between microglia and astrocyte in reactive gliosis following the CNS injury. Mol Neurobiol 48:690–701
Sofroniew MV (2005) Reactive astrocytes in neural repair and protection. Neuroscientist 11:400–407
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 97:11050–11055
Hepp DH, Foncke EMJ, Olde Dubbelink KTE, van de Berg WDJ, Berendse HW, Schoonheim MM (2017) Loss of functional connectivity in patients with Parkinson disease and visual hallucinations. Radiology 285:896–903
Shine JM, Halliday GM, Naismith SL, Lewis SJ (2011) Visual misperceptions and hallucinations in Parkinson’s disease: dysfunction of attentional control networks? Mov Disord 26:2154–2159
Stavitsky K, McNamara P, Durso R, Harris E, Auerbach S, Cronin-Golomb A (2008) Hallucinations, dreaming, and frequent dozing in Parkinson disease: impact of right-hemisphere neural networks. Cogn Behav Neurol 21:143–149
Jonas J, Frismand S, Vignal JP, Colnat-Coulbois S, Koessler L, Vespignani H, Rossion B, Maillard L (2014) Right hemispheric dominance of visual phenomena evoked by intracerebral stimulation of the human visual cortex. Hum Brain Mapp 35:3360–3371
Corbetta M, Patel G, Shulman GL (2008) The reorienting system of the human brain: from environment to theory of mind. Neuron 58:306–324
Huang L, Zhang D, Ji J, Wang Y, Zhang R (2021) Central retina changes in Parkinson’s disease: a systematic review and meta-analysis. J Neurol 268:4646–4654
Ortuno-Lizaran I, Beach TG, Serrano GE, Walker DG, Adler CH, Cuenca N (2018) Phosphorylated alpha-synuclein in the retina is a biomarker of Parkinson’s disease pathology severity. Mov Disord 33:1315–1324
Devos D, Tir M, Maurage CA, Waucquier N, Defebvre L, Defoort-Dhellemmes S, Destee A (2005) ERG and anatomical abnormalities suggesting retinopathy in dementia with Lewy bodies. Neurology 65:1107–1110
Langen CD, Cremers LGM, de Groot M, White T, Ikram MA, Niessen WJ, Vernooij MW (2018) Disconnection due to white matter hyperintensities is associated with lower cognitive scores. Neuroimage 183:745–756
Acknowledgements
The authors would like to thank all the participants involved in the study.
Funding
The Department of Health of the Basque Government through Project 2016111009 to J.C.G.E and I.G; M.D.C received a Post-doctoral research improvement fellowship from the Basque Government [POS_2018_1_0008].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
A.P.S. has been a consultant for Hoffman La Roche; received honoraria from GE Health Care Canada LTD, Hoffman La Roche. The rest of the authors declare no conflict of interest.
Ethics and informed consent
The present study was approved by the Euskadi Drug Research Ethics Committee (CEIm-E) (Approval number: PI2012154) and participants provided written informed consent prior to research participation.
Additional information
The original online version of this article was revised: An error in figure 1 introduced during typesetting while in production.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Diez-Cirarda, M., Cabrera-Zubizarreta, A., Murueta-Goyena, A. et al. Multimodal visual system analysis as a biomarker of visual hallucinations in Parkinson’s disease. J Neurol 270, 519–529 (2023). https://doi.org/10.1007/s00415-022-11427-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11427-x