Abstract
Background
Our earlier work showed that automaticity and retention of writing skills improved with intensive writing training in Parkinson’s disease (PD). However, whether this training changed the resting-state networks in the brain and how these changes underlie retention of motor learning is currently unknown.
Objective
To examine changes in resting-state functional connectivity (rs-FC) and their relation to behavioral changes immediately after writing training and at 6 week follow-up.
Methods
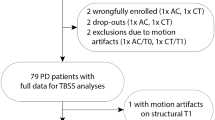
Twenty-five PD patients underwent resting-state fMRI (ON medication) before and after 6 weeks writing training. Motor learning was evaluated with a dual task paradigm pre- and post-training and at follow-up. Next, pre-post within-network changes in rs-FC were identified by an independent component analysis. Significant clusters were used as seeds in ROI-to-ROI analyses and rs-FC changes were correlated with changes in behavioral performance over time.
Results
Similar to our larger cohort findings, writing accuracy in single and dual task conditions improved post-training and this was maintained at follow-up. Connectivity within the dorsal attentional network (DAN) increased pre-post training, particularly with the right superior and middle temporal gyrus (rS/MTG). This cluster also proved more strongly connected to parietal and frontal areas and to cerebellar regions. Behavioral improvements from pre- to post-training and follow-up correlated with increased rs-FC between rS/MTG and the cerebellum.
Conclusions
Training-driven improvements in dual task writing led to functional reorganization within the DAN and increased connectivity with cerebellar areas. These changes were associated with the retention of writing gains and could signify task-specific neural changes or an inability to segregate neural networks.




Similar content being viewed by others
References
Abbruzzese G, Marchese R, Avanzino L, Pelosin E (2016) Rehabilitation for Parkinson’s disease: current outlook and future challenges. Parkinsonism Relat Disord 22:S60–S64. https://doi.org/10.1016/j.parkreldis.2015.09.005
Radder DLM, Silva L, de Lima A, Domingos J, Keus SHJ, van Nimwegen M, Bloem BR, de Vries NM (2020) Physiotherapy in Parkinson’s disease: a meta-analysis of present treatment modalities. Neurorehabil Neural Repair 34(10):871–880. https://doi.org/10.1177/1545968320952799
Bloem BR, Okun MS, Klein C (2021) Parkinson’s disease. Lancet 397(10291):2284–2303. https://doi.org/10.1016/S0140-6736(21)00218-X
Doyon J (2008) Motor sequence learning and movement disorders. Curr Opin Neurol 21:478–483. https://doi.org/10.1097/WCO.0b013e328304b6a3
Lehéricy S, Benali H, Van De Moortele PF, Pélégrini-Issac M, Waechter T, Ugurbil K, Doyon J (2005) Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci USA 102(35):12566–12571. https://doi.org/10.1073/pnas.0502762102
Marinelli L, Quartarone A, Hallett M, Frazzitta G, Ghilardi MF (2017) The many facets of motor learning and their relevance for Parkinson’s disease. Clin Neurophysiol 128(7):1127–1141. https://doi.org/10.1016/j.clinph.2017.03.042
Nieuwboer A, Rochester L, Müncks L, Swinnen SP (2009) Motor learning in Parkinson’s disease: limitations and potential for rehabilitation. Parkinsonism Relat Disord 15(Suppl. 3):53–58. https://doi.org/10.1016/S1353-8020(09)70781-3
Bernstein N (1967) The co-ordination and regulation of movements, 1st edn. Pergamon Press Ltd, Oxford
Wu T, Hallett M, Chan P (2015) Motor automaticity in Parkinson’s disease. Neurobiol Dis 82:226–234. https://doi.org/10.1016/j.nbd.2015.06.014
Bassett DS, Yang M, Wymbs NF, Grafton ST (2015) Learning-induced autonomy of sensorimotor systems. Nat Neurosci 18(5):744–751. https://doi.org/10.1038/nn.3993
Musslick S, Cohen JD (2021) Rationalizing constraints on the capacity for cognitive control. Trends Cogn Sci 25(9):757–775. https://doi.org/10.1016/j.tics.2021.06.001
Olson M, Lockhart TE, Lieberman A (2019) Motor learning deficits in Parkinson’s disease (PD) and their effect on training response in gait and balance: a narrative review. Front Neurol 10:1–17. https://doi.org/10.3389/fneur.2019.00062
Wu T, Liu J, Zhang H, Hallett M, Zheng Z, Chan P (2015) Attention to automatic movements in Parkinson’s disease: modified automatic mode in the striatum. Cereb Cortex 25(10):3330–3342. https://doi.org/10.1093/cercor/bhu135
Sarasso E, Agosta F, Piramide N, Gardoni A, Canu E, Leocadi M, Filippi M (2021) Action observation and motor imagery improve dual task in Parkinson’s disease: a clinical/fMRI study. Mov Disord 36(11):2569–2582. https://doi.org/10.1002/mds.28717
Nackaerts E, D’Cruz N, Dijkstra BW, Gilat M, Kramer T, Nieuwboer A (2019) Towards understanding neural network signatures of motor skill learning in Parkinson’s disease and healthy aging. Br J Radiol 92(1101):1–12. https://doi.org/10.1259/bjr.20190071
Hohenfeld C, Werner CJ, Reetz K (2018) Resting-state connectivity in neurodegenerative disorders: is there potential for an imaging biomarker? Neuroimage Clin 18:849–870. https://doi.org/10.1016/j.nicl.2018.03.013
Ma L, Narayana S, Robin DA, Fox PT, Xiong J (2011) Changes occur in resting state network of motor system during 4 weeks of motor skill learning. Neuroimage 58(1):226–233. https://doi.org/10.1016/j.neuroimage.2011.06.014
Solesio-Jofre E, Beets IAM, Woolley DG, Pauwels L, Chalavi S, Mantini D, Swinnen SP (2018) Age-dependent modulations of resting state connectivity following motor practice. Front Aging Neurosci 10:1–14. https://doi.org/10.3389/fnagi.2018.00025
Taubert M, Lohmann G, Margulies DS, Villringer A, Ragert P (2011) Long-term effects of motor training on resting-state networks and underlying brain structure. Neuroimage 57(4):1492–1498. https://doi.org/10.1016/j.neuroimage.2011.05.078
Cerasa A, Novellino F, Quattrone A (2016) Connectivity changes in Parkinson’s disease. Curr Neurol Neurosci Rep. https://doi.org/10.1007/s11910-016-0687-9
Tahmasian M, Eickhoff SB, Giehl K, Schwartz F, Herz DM, Drzezga A, Eickhoff CR (2017) Resting-state functional reorganization in Parkinson’s disease: an activation likelihood estimation meta-analysis. Cortex 92:119–138. https://doi.org/10.1016/j.cortex.2017.03.016
King LA, Mancini M, Smulders K, Harker G, Lapidus JA, Ramsey K, Horak FB (2020) Cognitively challenging agility boot camp program for freezing of gait in Parkinson disease. Neurorehabil Neural Repair 34(5):417–427. https://doi.org/10.1177/1545968320909331
Droby A, Maidan I, Jacob Y, Giladi N, Hausdorff JM, Mirelman A (2020) Distinct effects of motor training on resting-state functional networks of the brain in Parkinson’s disease. Neurorehabil Neural Repair 34(9):795–803. https://doi.org/10.1177/1545968320940985
Nackaerts E, Michely J, Heremans E, Swinnen SP, Smits-Engelsman BCM, Vandenberghe W, Nieuwboer A (2018) Training for micrographia alters neural connectivity in Parkinson’s disease. Front Neurosci 12(3):1–11. https://doi.org/10.3389/fnins.2018.00003
Wagle Shukla A, Ounpraseuth S, Okun MS, Gray V, Schwankhaus J, Metzer WS (2012) Micrographia and related deficits in Parkinson’s disease: a cross-sectional study. BMJ Open 2(3):1–7. https://doi.org/10.1136/bmjopen-2011-000628
Heremans E, Nackaerts E, Vervoort G, Broeder S, Swinnen SP, Nieuwboer A (2016) Impaired retention of motor learning of writing skills in patients with Parkinson’s disease with freezing of gait. PLoS One 11(2):1–13. https://doi.org/10.1371/journal.pone.0148933
Nackaerts E, Heremans E, Vervoort G, Smits-Engelsman BCM, Swinnen SP, Vandenberghe W, Nieuwboer A (2016) Relearning of writing skills in Parkinson’s disease after intensive amplitude training. Mov Disord 31(8):1209–1216. https://doi.org/10.1002/mds.26565
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55(3):181–184. https://doi.org/10.1136/jnnp.55.3.181
Hoehn M, Yahr M (1967) Parkinsonism: onset, progression and mortality. Neurology 17(5):427–442. https://doi.org/10.1212/wnl.17.5.427
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113. https://doi.org/10.1016/0028-3932(71)90067-4
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, LaPelle N (2008) Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23(15):2129–2170. https://doi.org/10.1002/mds.22340
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiat Res 12:189–198
Nackaerts E, Nieuwboer A, Broeder S, Swinnen S, Vandenberghe W, Heremans E (2018) Altered effective connectivity contributes to micrographia in patients with Parkinson’s disease and freezing of gait. J Neurol 265(2):336–347. https://doi.org/10.1007/s00415-017-8709-3
Chen CC, Granger CV, Peimer CA, Moy OJ, Wald S (2005) Manual ability measure (MAM-16): a preliminary report on a new patient-centred and task-oriented outcome measure of hand function. Journal of Hand Surgery 30(2):207–216. https://doi.org/10.1016/j.jhsb.2004.12.005
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x
Schade S, Mollenhauer B, Trenkwalder C (2020) Levodopa equivalent dose conversion factors: an updated proposal including opicapone and safinamide. Mov Disord Clin Practice 7(3):1–3. https://doi.org/10.1002/mdc3.12921
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25(15):2649–2653. https://doi.org/10.1002/mds.23429
Broeder S, Nackaerts E, Nieuwboer A, Smits-Engelsman BCM, Swinnen SP, Heremans E (2014) The effects of dual tasking on handwriting in patients with Parkinson’s disease. Neuroscience 263:193–202. https://doi.org/10.1016/j.neuroscience.2014.01.019
Van Drempt N, McCluskey A, Lannin NA (2011) Handwriting in healthy people aged 65 years and over. Aust Occup Ther J 58(4):276–286. https://doi.org/10.1111/j.1440-1630.2011.00923.x
Strouwen C, Centre N, Mu L, Sciences R, Bloem BR, Nieuwboer A (2016) Test-retest reliability of dual-task outcome measures in people with Parkinson’s disease. Phys Ther 96(8):1276–1286. https://doi.org/10.2522/ptj.20150244
Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, Gorgolewski KJ (2019) fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods 16(1):111–116. https://doi.org/10.1038/s41592-018-0235-4
Whitfield-Gabrieli S, Nieto-Castanon A (2012) Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity 2(3):125–141. https://doi.org/10.1089/brain.2012.0073
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17(2):825–841. https://doi.org/10.1006/nimg.2002.1132
Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Wolf DH (2013) An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage 64(1):240–256. https://doi.org/10.1016/j.neuroimage.2012.08.052
Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Milham MP (2013) A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage 76:183–201. https://doi.org/10.1016/j.neuroimage.2013.03.004
Parkes L, Fulcher B, Yücel M, Fornito A (2018) An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. Neuroimage 171:415–436. https://doi.org/10.1016/j.neuroimage.2017.12.073
Aquino KM, Fulcher BD, Parkes L, Sabaroedin K, Fornito A (2020) Identifying and removing widespread signal deflections from fMRI data: rethinking the global signal regression problem. Neuroimage 212:116614. https://doi.org/10.1016/j.neuroimage.2020.116614
Hyvärinen A (1999) Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans Neural Netw 10(3):626–634. https://doi.org/10.1109/72.761722
Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD (2011) Comparison of multi-subject ICA methods for analysis of fMRI data. Hum Brain Mapp 32(12):2075–2095. https://doi.org/10.1002/hbm.21170
Rothman KJ (1990) No adjustments are needed for multiple comparisons. Epidemiology 43–46(1):1
Hinkle DE, Wiersma W, Jurs SG (2003) Applied statistics for the behavioral sciences. Houghton Mifflin. Retrieved from https://books.google.be/books?id=7tntAAAAMAAJ
Heremans E, Broeder S, Nieuwboer A, Bekkers EM, Ginis P, Janssens L, Nackaerts E (2019) When motor control gets out of hand: speeding up triggers freezing in the upper limb in Parkinson’s disease. Parkinsonism Relat Disord 64:163–168. https://doi.org/10.1016/j.parkreldis.2019.04.005
Heremans E, Nackaerts E, Vervoort G, Vercruysse S, Broeder S, Strouwen C, Nieuwboer A (2015) Amplitude manipulation evokes upper limb freezing during handwriting in patients with Parkinson’s disease with freezing of gait. PLoS One 10(11):1–13. https://doi.org/10.1371/journal.pone.0142874
Nackaerts E, Nieuwboer A, Broeder S, Smits-Engelsman BCM, Swinnen SP, Vandenberghe W, Heremans E (2016) Opposite effects of visual cueing during writing-like movements of different amplitudes in Parkinson’s disease. Neurorehabil Neural Repair 30(5):431–439. https://doi.org/10.1177/1545968315601361
Wu T, Zhang J, Hallett M, Feng T, Hou Y, Chan P (2016) Neural correlates underlying micrographia in Parkinson’s disease. Brain 139(1):144–160. https://doi.org/10.1093/brain/awv319
Zham P, Poosapadi SA, Kempster P, Raghav S, Nagao KJ, Wong K, Kumar D (2021) Differences in levodopa response for progressive and non-progressive micrographia in Parkinson’s disease. Front Neurol 12:1–8. https://doi.org/10.3389/fneur.2021.665112
Vossel S, Geng JJ, Fink GR (2014) Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist 20(2):150–159. https://doi.org/10.1177/1073858413494269
Wu T, Chan P, Hallett M (2010) Effective connectivity of neural networks in automatic movements in Parkinson’s disease. Neuroimage 49(3):2581–2587. https://doi.org/10.1016/j.neuroimage.2009.10.051
Wu T, Hallett M (2008) Neural correlates of dual task performance in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 79(7):760–766. https://doi.org/10.1136/jnnp.2007.126599
Maidan I, Jacob Y, Giladi N, Hausdorff JM, Mirelman A (2019) Altered organization of the dorsal attention network is associated with freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord 63:77–82. https://doi.org/10.1016/j.parkreldis.2019.02.036
Danna J, Velay JL (2015) Basic and supplementary sensory feedback in handwriting. Front Psychol 6:1–11. https://doi.org/10.3389/fpsyg.2015.00169
Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME (2006) Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA 103(26):10046–10051. https://doi.org/10.1073/pnas.0604187103
Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102(27):9673–9678. https://doi.org/10.1073/pnas.0504136102
O’Callaghan C, Hornberger M, Balsters JH, Halliday GM, Lewis SJG, Shine JM (2016) Cerebellar atrophy in Parkinson’s disease and its implication for network connectivity. Brain 139(3):845–855. https://doi.org/10.1093/brain/awv399
Palmer WC, Cholerton BA, Zabetian CP, Montine TJ, Grabowski TJ, Rane S (2021) Resting-state cerebello-cortical dysfunction in Parkinson’s disease. Front Neurol 11:1–8. https://doi.org/10.3389/fneur.2020.594213
Wu T, Hallett M (2013) The cerebellum in Parkinson’s disease. Brain 136(3):696–709. https://doi.org/10.1093/brain/aws360
Festini SB, Bernard JA, Kwak Y, Peltier S, Bohnen NI, Müller MLTM, Seidler RD (2015) Altered cerebellar connectivity in Parkinson’s patients ON and OFF L-DOPA medication. Front Human Neurosci 9(1):1–13. https://doi.org/10.3389/fnhum.2015.00214
Brissenden JA, Levin EJ, Osher DE, Halko MA, Somers DC (2016) Functional evidence for a cerebellar node of the dorsal attention network. J Neurosci 36(22):6083–6096. https://doi.org/10.1523/JNEUROSCI.0344-16.2016
Buckner RL, Krienen FM, Castellanos A, Diaz JC, Thomas Yeo BT (2011) The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106(5):2322–2345. https://doi.org/10.1152/jn.00339.2011
Woolley DG, Wenderoth N, Heuninckx S, Zhang X, Callaert D, Swinnen SP (2010) Visual guidance modulates hemispheric asymmetries during an interlimb coordination task. Neuroimage 50(4):1566–1577. https://doi.org/10.1016/j.neuroimage.2010.01.012
Corbetta M, Patel G, Shulman GL (2008) The reorienting system of the human brain: from environment to theory of mind. Neuron 58(3):306–324. https://doi.org/10.1016/j.neuron.2008.04.017
Nackaerts E, Michely J, Heremans E, Swinnen S, Smits-Engelsman B, Vandenberghe W, Nieuwboer A (2018) Being on target: visual information during writing affects effective connectivity in Parkinson’s disease. Neuroscience 371:484–494. https://doi.org/10.1016/j.neuroscience.2017.12.027
Wu T, Liu J, Hallett M, Zheng Z, Chan P (2013) Cerebellum and integration of neural networks in dual-task processing. Neuroimage 65:466–475. https://doi.org/10.1016/j.neuroimage.2012.10.004
Guell X, Schmahmann J (2020) Cerebellar functional anatomy: a didactic summary based on human fMRI evidence. Cerebellum 19(1):1–5. https://doi.org/10.1007/s12311-019-01083-9
van Es DM, van der Zwaag W, Knapen T (2019) Topographic maps of visual space in the human cerebellum. Curr Biol 29(10):1689-1694.e3. https://doi.org/10.1016/j.cub.2019.04.012
Acknowledgements
We are grateful to all participants in this study. We thank Dr. Bruno Bergmans (AZ Sint-Jan, Bruges) for his help in recruitment of participants and Ir. Marc Beirinckx for development of the tablet and for providing technical support.
Funding
The Research Foundation Flanders (FWO) [grant number G.0906.11 and G0A5619N] and the King Baudouin Foundation (Fund Druwé-Eerdekens 2018) supported this work. EN is a postdoctoral fellow funded by the Research Foundation Flanders (FWO) [grant number 12F4719N]. JDV is a doctoral fellow funded by the Research Foundation Flanders (FWO) [grant number 11N5622N].
Author information
Authors and Affiliations
Contributions
All authors contributed to the final manuscript of this study. Conceptualization, methodology and data collection were completed by EN. Formal data analysis and investigation were performed by JDV and EN. EN wrote the first draft of the manuscript. Review and editing of the manuscript were carried out by all co-authors. AN was responsible for the overall supervision.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no relevant financial or non-financial interests to disclose.
Ethics approval
The study was approved by the local Ethics Committee Research UZ /KU Leuven (S54132) in accordance with the Declaration of Helsinki (1967).
Consent to participate
A written informed consent was obtained from all individual participants included in the study, after detailed explanation of the protocol and before the start of the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
De Vleeschhauwer, J., Nackaerts, E., D’Cruz, N. et al. Associations between resting-state functional connectivity changes and prolonged benefits of writing training in Parkinson’s disease. J Neurol 269, 4696–4707 (2022). https://doi.org/10.1007/s00415-022-11098-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11098-8