Abstract
Background
Amyotrophic lateral sclerosis (ALS) is an incurable neurological disease, and patients diagnosed with ALS have a survival time of 2–5 years without life-sustaining therapy. Decision-making processes for the acceptance or decline of percutaneous endoscopic gastrostomy (PEG) and tracheostomy with invasive ventilation (TIV) therapy are complex and multifaceted. In this study, we examined whether participation or no participation in clinical trials of ALS had an influence on the decision-making processes of ALS patients.
Methods
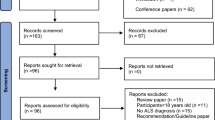
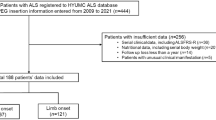
Fifty-seven consecutive ALS participants were recruited. Two participants did not wish to participate in any clinical trials, and Twenty-two participants were enrolled in clinical trials. Twenty-three participants wished to participate but could not be enrolled in any of the clinical trials because they exceeded the number of participants in these trials or they met the exclusion criteria.
Result
At baseline, there was no significant difference in the preference rates for PEG and TIV between the participant and non-participant groups, but after the double-blind period/6 months, both preference rates were significantly higher in the non-participant group than in the participant group. Notably, the rate of preferred TIV in the participant group drastically decreased after the double-blind period. A single regression analysis revealed that participation in clinical trials had a strong influence on the change of TIV preference for 6 months.
Conclusion
Participation in a clinical trial decreases the willingness to prolong life after the clinical trial. The present results are meaningful when designing clinical trials and discussing life-sustaining treatments with ALS patients.

Similar content being viewed by others
Availability of data and materials
Data are available for collaborative studies with qualified investigators after inquiry.
References
Charcot JM (1880) De la sclérose latérale amyotrophique - Symptomatologie Leçons sur les maladies du système nerveux, faites à la Salpêtrière. Delahaye, Paris
Charcot JM (1887–1888) Les Leçons du Mardi: Policlinique. Paris: Bureaux du Progrès Médical.
Goetz CG (2000) Amyotrophic lateral sclerosis: early contributions of Jean-Martin Charcot. Muscle Nerve 23:336–343
Jaiswal MK (2019) Riluzole and edaravone: a tale of two amyotrophic lateral sclerosis drugs. Med Res Rev 39:733–748
Rothstein JD (2017) Edaravone: a new drug approved for ALS. Cell 171:725
Brown RH, Al-Chalabi A (2017) Amyotrophic lateral sclerosis. N Engl J Med 377:162–172
Hayashi N, Atsuta N, Yokoi D, Nakamura R, Nakatochi M, Katsuno M, Izumi Y, Kanai K, Hattori N, Taniguchi A, Morita M, Kano O, Shibuya K, Kuwabara S, Suzuki N, Aoki M, Aiba I, Mizoguchi K, Oda M, Kaji R, Sobue G (2020) Prognosis of amyotrophic lateral sclerosis patients undergoing tracheostomy invasive ventilation therapy in Japan. J Neurol Neurosurg Psychiatry 91:285–290
Neudert C, Oliver D, Wasner M, Borasio GD (2001) The course of the terminal phase in patients with amyotrophic lateral sclerosis. J Neurol 248:612–616
Andersen PM, Kuzma-Kozakiewicz M, Keller J, Aho-Oezhan HEA, Ciecwierska K, Szejko N, Vazquez C, Bohm S, Badura-Lotter G, Meyer T, Petri S, Linse K, Hermann A, Semb O, Stenberg E, Nackberg S, Dorst J, Uttner I, Haggstrom AC, Ludolph AC, Lule D (2018) Therapeutic decisions in ALS patients: cross-cultural differences and clinical implications. J Neurol 265:1600–1606
Ritsma BR, Berger MJ, Charland DA, Khoury MA, Phillips JT, Quon MJ, Strong MJ, Schulz VM (2010) NIPPV: prevalence, approach and barriers to use at Canadian ALS centres. Can J Neurol Sci 37:54–60
Lechtzin N, Wiener CM, Clawson L, Davidson MC, Anderson F, Gowda N, Diette GB, Group ACS (2004) Use of noninvasive ventilation in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 5:9–15
Chio A, Calvo A, Ghiglione P, Mazzini L, Mutani R, Mora G, Parals (2010) Tracheostomy in amyotrophic lateral sclerosis: a 10-year population-based study in Italy. J Neurol Neurosurg Psychiatry 81:1141–1143
Tagami M, Kimura F, Nakajima H, Ishida S, Fujiwara S, Doi Y, Hosokawa T, Yamane K, Unoda K, Hirose T, Tani H, Ota S, Ito T, Sugino M, Shinoda K, Hanafusa T (2014) Tracheostomy and invasive ventilation in Japanese ALS patients: decision-making and survival analysis: 1990–2010. J Neurol Sci 344:158–164
Vianello A, Concas A (2014) Tracheostomy ventilation in ALS: a Japanese bias. J Neurol Sci 344:3–4
Kurisaki R, Yamashita S, Sakamoto T, Maruyoshi N, Uekawa K, Uchino M, Ando Y (2014) Decision making of amyotrophic lateral sclerosis patients on noninvasive ventilation to receive tracheostomy positive pressure ventilation. Clin Neurol Neurosurg 125:28–31
Rabkin J, Ogino M, Goetz R, McElhiney M, Hupf J, Heitzman D, Heiman-Patterson T, Miller R, Katz J, Lomen-Hoerth C, Imai T, Atsuta N, Morita M, Tateishi T, Matsumura T, Mitsumoto H (2014) Japanese and American ALS patient preferences regarding TIV (tracheostomy with invasive ventilation): a cross-national survey. Amyotroph Lateral Scler Frontotemporal Degener 15:185–191
Greenaway LP, Martin NH, Lawrence V, Janssen A, Al-Chalabi A, Leigh PN, Goldstein LH (2015) Accepting or declining non-invasive ventilation or gastrostomy in amyotrophic lateral sclerosis: patients’ perspectives. J Neurol 262:1002–1013
Christodoulou G, Goetz R, Ogino M, Mitsumoto H, Rabkin J (2015) Opinions of Japanese and American ALS caregivers regarding tracheostomy with invasive ventilation (TIV). Amyotroph Lateral Scler Frontotemporal Degener 17:47–54
Morimoto S, Takahashi S, Fukushima K, Saya H, Suzuki N, Aoki M, Okano H, Nakahara J (2019) Ropinirole hydrochloride remedy for amyotrophic lateral sclerosis - Protocol for a randomized, double-blind, placebo-controlled, single-center, and open-label continuation phase I/IIa clinical trial (ROPALS trial). Regen Ther 11:143–166
Takahashi S (2020) Phase 1/2a, Double-blind, Placebo-controlled Study with an Open-label Extension of Ropinirole Hydrochloride Extended-Release Tablets-Explorative Assessment of the Safety, Tolerability, and Efficacy after Oral Treatment in Patients with Amyotrophic Lateral Sclerosis (ALS) https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000039856. Accessed 21 Nov 2021
Okano H, Yasuda D, Fujimori K, Morimoto S, Takahashi S (2020) Ropinirole, a new ALS drug candidate developed using iPSCs. Trends Pharmacol Sci 41:99–109
Biogen (2021) An Efficacy, Safety, Tolerability, Pharmacokinetics and Pharmacodynamics Study of BIIB067 in Adults With Inherited Amyotrophic Lateral Sclerosis (ALS) (VALOR (Part C)) https://clinicaltrials.gov/ct2/show/NCT02623699. Accessed 24 Mar 2021
Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron D (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299
Team RC (2021) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Acknowledgements
The authors would like to thank the staff of Keio University Hospital.
Funding
This research was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 21H02812) (to DI), Foundation of Japan Amyotrophic Lateral Sclerosis Association, Ice Bucket Challenge Grant (Japan Amyotrophic Lateral Sclerosis Association) (to DI and HO), Serika Fund (to DI and HO) and a grant support from Japan Agency for Medical Research and Development [Research on Practical Application of Innovative Pharmaceutical and Medical Devices for Rare and Intractable Diseases (Grant No. JP 18ek0109395, JP 19ek0109395, JP 20ek0109395, JP 18ek0109329, JP 19ek0109329, JP 20ek0109329) to HO].
Author information
Authors and Affiliations
Contributions
Study conception and design were conducted by DI. Data processing and statistical analyses were conducted by CK, SM, ST, YD, KO, HO, and JN. The first draft of the manuscript was written by CK, and all authors reviewed, critiqued, and edited subsequent versions of the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest, except that HO is a founder scientist and a Scientific Advisory Board of SanBio Co. Ltd. and K Pharma Inc and DI is the principal investigator of the VALOR study [ClinicalTrials.gov Identifier: NCT02623699].
Ethical approval
The study design and protocol were approved by the Ethics Committee for Human Research of the Keio University School of Medicine (#20200287).
Rights and permissions
About this article
Cite this article
Kato, C., Morimoto, S., Takahashi, S. et al. Influence of a clinical trial in the decision-making processes of patients with amyotrophic lateral sclerosis. J Neurol 269, 2634–2640 (2022). https://doi.org/10.1007/s00415-021-10862-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10862-6