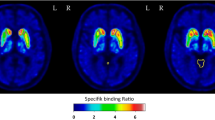
Abstract
Although the diagnosis of Parkinson’s disease (PD) is essentially clinical, the implementation of imaging techniques can improve diagnostic accuracy. While some techniques (e.g. magnetic resonance imaging—MRI, computerized tomography—CT) are used to exclude secondary syndromes, presynaptic dopaminergic imaging including imaging of dopamine transporter (DAT)—can help the Neurologist in the differential diagnosis between neurodegenerative parkinsonian syndromes and parkinsonism without dopamine deficiency. DAT imaging can be useful in cases in which the clinical picture is not univocal, as in case of overlapping clinical features in patients with early disease, atypical syndromes or unsatisfying response to therapy. Currently, (123I)FP-CIT ([123I]N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl)nortropane) (trade name DaTSCAN) is the only agent approved by international regulatory agencies for this purpose. With the increasing use of this technique, some unexpected findings have been reported, including patients clinically diagnosed with PD with a normal SPECT scan [e.g. Scans Without Evidence of Dopaminergic Deficit (SWEDD)]; PD patients with a greater dopaminergic deficit in the striatum ipsilateral to the clinically more affected side [e.g. Scans With Ipsilateral Dopaminergic Deficit (SWIDD)]; as well as some artifacts. Moreover, the neurologist must remember that structural lesions and administration of some drugs might alter the result of DAT imaging. Unexpected findings, artifacts, and misinterpretation of imaging findings can lead to an erroneous diagnosis and inappropriate therapy, neglect of other medical conditions that might explain the clinical picture, and undermine the selection phase in clinical trials. The aim of the present review is to bring clarity on these controversial (and sometimes erroneous) results, in order to inform of these possibilities the clinicians requesting a DaTSCAN in clinical practice.


Similar content being viewed by others
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Change history
09 December 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00415-021-10923-w
References
Postuma RB, Berg D, Stern M et al (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30:1591–1601. https://doi.org/10.1002/mds.26424
Parnetti L, Gaetani L, Eusebi P et al (2019) CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol 18:573–586. https://doi.org/10.1016/S1474-4422(19)30024-9
Caminiti SP, Alongi P, Majno L et al (2017) Evaluation of an optimized [18F]fluoro-deoxy-glucose positron emission tomography voxel-wise method to early support differential diagnosis in atypical Parkinsonian disorders. Eur J Neurol 24:687-e26. https://doi.org/10.1111/ene.13269
Pilotto A, Premi E, Paola Caminiti S et al (2018) Single-subject SPM FDG-PET patterns predict risk of dementia progression in Parkinson disease. Neurology 90:e1029–e1037. https://doi.org/10.1212/WNL.0000000000005161
Hellwig S, Amtage F, Kreft A et al (2012) [18F]FDG-PET is superior to [123I]IBZM-SPECT for the differential diagnosis of parkinsonism. Neurology 79:1314–1322. https://doi.org/10.1212/WNL.0b013e31826c1b0a
Morbelli S, Esposito G, Arbizu J et al (2020) EANM practice guideline/SNMMI procedure standard for dopaminergic imaging in Parkinsonian syndromes 1.0. Eur J Nucl Med Mol Imaging 47:1885–1912. https://doi.org/10.1007/s00259-020-04817-8
Leung K (2004) N-(3-Fluoropropyl)-2β-carbomethoxy-3β-(4-[123I]iodophenyl)nortropane. Molecular imaging and contrast agent database (MICAD). National Center for Biotechnology Information (US), Bethesda (MD)
Colloby SJ, McParland S, O’Brien JT, Attems J (2012) Neuropathological correlates of dopaminergic imaging in Alzheimer’s disease and Lewy body dementias. Brain J Neurol 135:2798–2808. https://doi.org/10.1093/brain/aws211
Kraemmer J, Kovacs GG, Perju-Dumbrava L et al (2014) Correlation of striatal dopamine transporter imaging with post mortem substantia nigra cell counts. Mov Disord 29:1767–1773. https://doi.org/10.1002/mds.25975
Honkanen EA, Saari L, Orte K et al (2019) No link between striatal dopaminergic axons and dopamine transporter imaging in Parkinson’s disease. Mov Disord 34:1562–1566. https://doi.org/10.1002/mds.27777
Saari L, Kivinen K, Gardberg M et al (2017) Dopamine transporter imaging does not predict the number of nigral neurons in Parkinson disease. Neurology 88:1461–1467. https://doi.org/10.1212/WNL.0000000000003810
Perlmutter JS, Stoessl AJ (2019) Striatal DAT SPECT: caveat emptor! Mov Disord 34:1430–1432. https://doi.org/10.1002/mds.27811
Brigo F, Matinella A, Erro R, Tinazzi M (2014) [123I]FP-CIT SPECT (DaTSCAN) may be a useful tool to differentiate between Parkinson’s disease and vascular or drug-induced parkinsonisms: a meta-analysis. Eur J Neurol 21:1369-e90. https://doi.org/10.1111/ene.12444
Saeed U, Compagnone J, Aviv RI et al (2017) Imaging biomarkers in Parkinson’s disease and Parkinsonian syndromes: current and emerging concepts. Transl Neurodegener 6:8. https://doi.org/10.1186/s40035-017-0076-6
Benamer HTS, Oertel WH, Patterson J et al (2003) Prospective study of presynaptic dopaminergic imaging in patients with mild parkinsonism and tremor disorders: part 1. Baseline and 3 month observations. Mov Disord 18:977–984. https://doi.org/10.1002/mds.10482
Fahn S, Oakes D, Shoulson I et al (2004) Levodopa and the progression of Parkinson’s disease. N Engl J Med 351:2498–2508. https://doi.org/10.1056/NEJMoa033447
Parkinson Progression Marker Initiative (2011) The Parkinson progression marker initiative (PPMI). Prog Neurobiol 95:629–635. https://doi.org/10.1016/j.pneurobio.2011.09.005
Fahn S, Group and the PS (2005) Does levodopa slow or hasten the rate of progression of Parkinson’s disease? J Neurol 252:iv37–iv42. https://doi.org/10.1007/s00415-005-4008-5
Whone AL, Watts RL, Stoessl AJ et al (2003) Slower progression of Parkinson’s disease with ropinirole versus levodopa: the REAL-PET study. Ann Neurol 54:93–101. https://doi.org/10.1002/ana.10609
Erro R, Schneider SA, Stamelou M et al (2016) What do patients with scans without evidence of dopaminergic deficit (SWEDD) have? New evidence and continuing controversies. J Neurol Neurosurg Psychiatry 87:319–323. https://doi.org/10.1136/jnnp-2014-310256
Cilia R, Reale C, Castagna A et al (2014) Novel DYT11 gene mutation in patients without dopaminergic deficit (SWEDD) screened for dystonia. Neurology 83:1155–1162. https://doi.org/10.1212/WNL.0000000000000821
Rosa AD, Carducci C, Carducci C et al (2014) Screening for dopa-responsive dystonia in patients with scans without evidence of dopaminergic deficiency (SWEDD). J Neurol 261:2204–2208. https://doi.org/10.1007/s00415-014-7477-6
Schneider SA, Edwards MJ, Mir P et al (2007) Patients with adult-onset dystonic tremor resembling parkinsonian tremor have scans without evidence of dopaminergic deficit (SWEDDs). Mov Disord 22:2210–2215. https://doi.org/10.1002/mds.21685
Schwingenschuh P, Ruge D, Edwards MJ et al (2010) Distinguishing SWEDDs patients with asymmetric resting tremor from Parkinson’s disease: a clinical and electrophysiological study. Mov Disord 25:560–569. https://doi.org/10.1002/mds.23019
Cáceres-Redondo MT, Carrillo F, Palomar FJ, Mir P (2012) DYT-1 gene dystonic tremor presenting as a “scan without evidence of dopaminergic deficit.” Mov Disord 27:1469–1469. https://doi.org/10.1002/mds.25171
de Laat KF, van de Warrenburg BP (2012) Re-emergent tremor in a dystonic SWEDD case. Mov Disord 27:462–463. https://doi.org/10.1002/mds.24040
Stockner H, Schwingenschuh P, Djamshidian A et al (2012) Is transcranial sonography useful to distinguish scans without evidence of dopaminergic deficit patients from Parkinson’s disease? Mov Disord 27:1182–1185. https://doi.org/10.1002/mds.25102
Benamer TS, Patterson J, Grosset DG et al (2000) Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: the [123I]-FP-CIT study group. Mov Disord 15:503–510
Booij J, Speelman JD, Horstink MWIM, Wolters EC (2001) The clinical benefit of imaging striatal dopamine transporters with [123I]FP-CIT SPET in differentiating patients with presynaptic parkinsonism from those with other forms of parkinsonism. Eur J Nucl Med 28:266–272. https://doi.org/10.1007/s002590000460
Eckert T, Feigin A, Lewis DE et al (2007) Regional metabolic changes in Parkinsonian patients with normal dopaminergic imaging. Mov Disord 22:167–173. https://doi.org/10.1002/mds.21185
Jennings DL, Seibyl JP, Oakes D et al (2004) (123I) beta-CIT and single-photon emission computed tomographic imaging vs clinical evaluation in Parkinsonian syndrome: unmasking an early diagnosis. Arch Neurol 61:1224–1229. https://doi.org/10.1001/archneur.61.8.1224
Marshall VL, Reininger CB, Marquardt M et al (2009) Parkinson’s disease is overdiagnosed clinically at baseline in diagnostically uncertain cases: a 3 year European multicenter study with repeat [123I]FP-CIT SPECT. Mov Disord 24:500–508. https://doi.org/10.1002/mds.22108
Serrano Vicente J, García Bernardo L, Durán Barquero C et al (2009) Valor predictivo negativo del SPECT con Ioflupano 123I en los trastornos del movimiento. Rev Esp Med Nucl 28:2–5. https://doi.org/10.1016/S0212-6982(09)70207-1
Sixel-Döring F, Liepe K, Mollenhauer B et al (2011) The role of 123I-FP-CIT-SPECT in the differential diagnosis of Parkinson and tremor syndromes: a critical assessment of 125 cases. J Neurol 258:2147–2154. https://doi.org/10.1007/s00415-011-6076-z
Bajaj NPS, Wang L, Gontu V et al (2012) Accuracy of subjective and objective handwriting assessment for differentiating Parkinson’s disease from tremulous subjects without evidence of dopaminergic deficits (SWEDDs): an FP-CIT-validated study. J Neurol 259:2335–2340. https://doi.org/10.1007/s00415-012-6495-5
Utiumi MAT, Felício AC, Borges CR et al (2012) Dopamine transporter imaging in clinically unclear cases of parkinsonism and the importance of scans without evidence of dopaminergic deficit (SWEDDs). Arq Neuropsiquiatr 70:667–673. https://doi.org/10.1590/S0004-282X2012000900004
Marek K, Seibyl J, Eberly S et al (2014) Longitudinal follow-up of SWEDD subjects in the PRECEPT study. Neurology 82:1791–1797. https://doi.org/10.1212/WNL.0000000000000424
Menéndez-González M, Tavares F, Zeidan N et al (2014) Diagnoses behind patients with hard-to-classify tremor and normal DaT-SPECT: a clinical follow up study. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2014.00056
Menéndez-González M, Álvarez-Avellón T, Salas-Pacheco JM et al (2018) Frontotemporal lobe degeneration as origin of scans without evidence of dopaminergic deficit. Front Neurol 9:335. https://doi.org/10.3389/fneur.2018.00335
Lee JW, Song YS, Kim H et al (2021) Patients with scans without evidence of dopaminergic deficit (SWEDD) do not have early Parkinson’s disease: analysis of the PPMI data. PLoS ONE 16:e0246881. https://doi.org/10.1371/journal.pone.0246881
Badzek S, Miletic V, Prejac J et al (2013) Paraneoplastic stiff person syndrome associated with colon cancer misdiagnosed as idiopathic Parkinson’s disease worsened after capecitabine therapy. World J Surg Oncol 11:224. https://doi.org/10.1186/1477-7819-11-224
Jakobson Mo S, Linder J, Forsgren L, Riklund K (2015) Accuracy of visual assessment of dopamine transporter imaging in early parkinsonism. Mov Disord Clin Pract 2:17–23. https://doi.org/10.1002/mdc3.12089
Hermida AP, Janjua AU, Glass OM et al (2016) A case of lithium-induced parkinsonism presenting with typical motor symptoms of Parkinson’s disease in a bipolar patient. Int Psychogeriatr 28:2101–2104. https://doi.org/10.1017/S1041610216001101
Bain PG (2009) Dystonic tremor presenting as parkinsonism: long term follow-up of SWEDD. Neurology 72:1443–1445. https://doi.org/10.1212/WNL.0b013e3181a18809
Kwon D-Y, Kwon Y, Kim J-W (2018) Quantitative analysis of finger and forearm movements in patients with off state early stage Parkinson’s disease and scans without evidence of dopaminergic deficit (SWEDD). Parkinsonism Relat Disord 57:33–38. https://doi.org/10.1016/j.parkreldis.2018.07.012
Mian OS, Schneider SA, Schwingenschuh P et al (2011) Gait in SWEDDs patients: comparison with Parkinson’s disease patients and healthy controls. Mov Disord 26:1266–1273. https://doi.org/10.1002/mds.23684
Zheng H-G, Zhang R, Li X et al (2015) Heterogeneity of monosymptomatic resting tremor in a prospective study: clinical features, electrophysiological test, and dopamine transporter positron emission tomography. Chin Med J (Engl) 128:1765–1771. https://doi.org/10.4103/0366-6999.159352
Suwijn SR, Samim H, Eggers C et al (2020) Value of clinical signs in identifying patients with scans without evidence of dopaminergic deficit (SWEDD). J Park Dis 10:1561–1569. https://doi.org/10.3233/JPD-202090
Bajaj NPS, Gontu V, Birchall J et al (2010) Accuracy of clinical diagnosis in tremulous parkinsonian patients: a blinded video study. J Neurol Neurosurg Psychiatry 81:1223–1228. https://doi.org/10.1136/jnnp.2009.193391
Jang W, Kim J-S, Cho JW et al (2013) Cardiac sympathetic denervation in Parkinson’s disease patients with SWEDDs. Neurol Sci 34:1375–1382. https://doi.org/10.1007/s10072-012-1244-1
Yang H-J, Kim YE, Yun JY et al (2014) Comparison of sleep and other non-motor symptoms between SWEDDs patients and de novo Parkinson’s disease patients. Parkinsonism Relat Disord 20:1419–1422. https://doi.org/10.1016/j.parkreldis.2014.09.024
Jackson L, Turcano P, Stitt D et al (2020) Autonomic testing profiles in scans without evidence of dopaminergic deficit (SWEDD). J Park Dis 10:945–949. https://doi.org/10.3233/JPD-201944
Oh Y-S, Choi JH, Kwon D-Y (2016) Classification of scans without evidence of dopamine deficit (SWEDD) according to the olfactory function. J Park Dis 6:771–778. https://doi.org/10.3233/JPD-160874
Sprenger FS, Seppi K, Djamshidian A et al (2015) Nonmotor symptoms in subjects without evidence of dopaminergic deficits. Mov Disord 30:976–981. https://doi.org/10.1002/mds.26204
Taylor S, Gafton J, Shah B et al (2016) Progression of nonmotor symptoms in subgroups of patients with non–dopamine-deficient Parkinsonism. Mov Disord 31:344–351. https://doi.org/10.1002/mds.26456
Kurlawala Z, Shadowen PH, McMillan JD et al (2021) Progression of nonmotor symptoms in Parkinson’s disease by sex and motor laterality. Park Dis 2021:1–12. https://doi.org/10.1155/2021/8898887
Yu Z, Li Y (2021) Association of autonomic symptoms with cerebrospinal fluid biomarkers in Parkinson disease and scans without evidence of dopaminergic deficit. Medicine (Baltimore) 100:e24837. https://doi.org/10.1097/MD.0000000000024837
Wyman-Chick KA, Martin PK, Minár M, Schroeder RW (2016) Cognition in patients with a clinical diagnosis of Parkinson disease and scans without evidence of dopaminergic deficit (SWEDD): 2 year follow-up. Cogn Behav Neurol 29:190–196. https://doi.org/10.1097/WNN.0000000000000107
Wyman-Chick KA, Martin PK, Minár M et al (2018) Neuropsychological test performance in parkinsonism without dopaminergic deficiency on [123I]-FP-CIT SPECT imaging. J Int Neuropsychol Soc 24:646–651. https://doi.org/10.1017/S1355617718000164
Silveira-Moriyama L, Birchall J, Bain P et al (2015) Hyposmia in SWEDD: letter to the editors. Mov Disord 30:1436–1437. https://doi.org/10.1002/mds.26344
Leger C, Herbert M, DeSouza JFX (2020) Non-motor clinical and biomarker predictors enable high cross-validated accuracy detection of early PD but lesser cross-validated accuracy detection of scans without evidence of dopaminergic deficit. Front Neurol 11:364. https://doi.org/10.3389/fneur.2020.00364
Nicastro N, Burkhard PR, Garibotto V (2020) Preserved extrastriatal 123I-FP-CIT binding in scans without evidence of dopaminergic deficit (SWEDD). Mol Imaging Biol 22:1592–1599. https://doi.org/10.1007/s11307-020-01502-y
Kim M, Park H (2016) Structural connectivity profile of scans without evidence of dopaminergic deficit (SWEDD) patients compared to normal controls and Parkinson’s disease patients. Springerplus 5:1421. https://doi.org/10.1186/s40064-016-3110-8
Kim M, Park H (2016) Using tractography to distinguish SWEDD from Parkinson’s disease patients based on connectivity. Park Dis 2016:8704910. https://doi.org/10.1155/2016/8704910
Jin L, Zeng Q, He J et al (2019) A ReliefF-SVM-based method for marking dopamine-based disease characteristics: a study on SWEDD and Parkinson’s disease. Behav Brain Res 356:400–407. https://doi.org/10.1016/j.bbr.2018.09.003
Feng Y, Yan W, Wang J et al (2020) Local white matter fiber clustering differentiates Parkinson’s disease diagnoses. Neuroscience 435:146–160. https://doi.org/10.1016/j.neuroscience.2020.03.049
Kang S-J, Ahn JY, Kim J-S et al (2016) 24-hour ambulatory blood pressure monitoring in SWEDDs patients With parkinsonism. Can J Neurol Sci 43:390–397. https://doi.org/10.1017/cjn.2015.385
Yoshii F, Ryo M, Baba Y et al (2017) Combined use of dopamine transporter imaging (DAT-SPECT) and 123I-metaiodobenzylguanidine (MIBG) myocardial scintigraphy for diagnosing Parkinson’s disease. J Neurol Sci 375:80–85. https://doi.org/10.1016/j.jns.2017.01.042
Qamhawi Z, Towey D, Shah B et al (2015) Clinical correlates of raphe serotonergic dysfunction in early Parkinson’s disease. Brain 138:2964–2973. https://doi.org/10.1093/brain/awv215
Schirinzi T, Di Lorenzo F, Ponzo V et al (2016) Mild cerebello-thalamo-cortical impairment in patients with normal dopaminergic scans (SWEDD). Parkinsonism Relat Disord 28:23–28. https://doi.org/10.1016/j.parkreldis.2016.03.023
Marshall VL, Patterson J, Hadley DM et al (2006) Two-year follow-up in 150 consecutive cases with normal dopamine transporter imaging. Nucl Med Commun 27:933–937. https://doi.org/10.1097/01.mnm.0000243374.11260.5b
Batla A, Erro R, Stamelou M et al (2014) Patients with scans without evidence of dopaminergic deficit: a long-term follow-up study. Mov Disord 29:1820–1825. https://doi.org/10.1002/mds.26018
van Dyck CH, Seibyl JP, Malison RT et al (2002) Age-related decline in dopamine transporters: analysis of striatal subregions, nonlinear effects, and hemispheric asymmetries. Am J Geriatr Psychiatry 10:36–43
Ross GW, Petrovitch H, Abbott RD et al (2004) Parkinsonian signs and substantia nigra neuron density in decendents elders without PD. Ann Neurol 56:532–539. https://doi.org/10.1002/ana.20226
Nicastro N, Garibotto V, Badoud S, Burkhard PR (2016) Scan without evidence of dopaminergic deficit: a 10 year retrospective study. Parkinsonism Relat Disord 31:53–58. https://doi.org/10.1016/j.parkreldis.2016.07.002
Nicastro N, Burkhard PR, Garibotto V (2018) Scan without evidence of dopaminergic deficit (SWEDD) in degenerative parkinsonism and dementia with Lewy bodies: a prospective study. J Neurol Sci 385:17–21. https://doi.org/10.1016/j.jns.2017.11.039
Wile DJ, Dinelle K, Vafai N et al (2016) A scan without evidence is not evidence of absence: scans without evidence of dopaminergic deficit in a symptomatic leucine-rich repeat kinase 2 mutation carrier. Mov Disord 31:405–409. https://doi.org/10.1002/mds.26450
Garibotto V, Nicastro N, Burkhard PR (2016) No more SWEDDs. Mov Disord 31:1426–1426. https://doi.org/10.1002/mds.26705
Garrido A, Iranzo A, Stefani A et al (2020) Lack of asymmetry of nigrostriatal dopaminergic function in healthy subjects. Mov Disord 35:1072–1076. https://doi.org/10.1002/mds.28019
Ling H, Kearney S, Yip HLK et al (2016) Parkinson’s disease without nigral degeneration: a pathological correlate of scans without evidence of dopaminergic deficit (SWEDD)? J Neurol Neurosurg Psychiatry 87:633–641. https://doi.org/10.1136/jnnp-2015-310756
Cilia R, Rossi C, Frosini D et al (2011) Dopamine transporter SPECT imaging in corticobasal syndrome. PLoS One. https://doi.org/10.1371/journal.pone.0018301
Kaasinen V, Gardberg M, Röyttä M et al (2013) Normal dopamine transporter SPECT in neuropathologically confirmed corticobasal degeneration. J Neurol 260:1410–1411. https://doi.org/10.1007/s00415-013-6886-2
McKinley J, O’Connell M, Farrell M, Lynch T (2014) Normal dopamine transporter imaging does not exclude multiple system atrophy. Parkinsonism Relat Disord 20:933–934. https://doi.org/10.1016/j.parkreldis.2014.04.022
O’Sullivan SS, Burn DJ, Holton JL, Lees AJ (2008) Normal dopamine transporter single photon-emission CT scan in corticobasal degeneration. Mov Disord 23:2424–2426. https://doi.org/10.1002/mds.22323
van der Zande JJ, Booij J, Scheltens P et al (2016) [(123)]FP-CIT SPECT scans initially rated as normal became abnormal over time in patients with probable dementia with Lewy bodies. Eur J Nucl Med Mol Imaging 43:1060–1066. https://doi.org/10.1007/s00259-016-3312-x
Kolenc M, Popović M, Grmek M et al (2012) A case of multiple system atrophy with normal dopamine transporter imaging. J Neurol 259:2729–2731. https://doi.org/10.1007/s00415-012-6643-y
Muñoz E, Iranzo A, Rauek S et al (2011) Subclinical nigrostriatal dopaminergic denervation in the cerebellar subtype of multiple system atrophy (MSA-C). J Neurol 258:2248–2253. https://doi.org/10.1007/s00415-011-6108-8
Vergnet S, Hives F, Foubert-Samier A et al (2019) Dopamine transporter imaging for the diagnosis of multiple system atrophy cerebellar type. Parkinsonism Relat Disord 63:199–203. https://doi.org/10.1016/j.parkreldis.2019.02.006
Aguirregomozcorta M, Stamelou M, Antonini A et al (2013) Patients with rest-tremor and scans with ipsilateral dopaminergic deficit. J Neurol 260:1132–1135. https://doi.org/10.1007/s00415-012-6774-1
Hoshiyama E, Kadowaki T, Nakamura A et al (2015) The decreasing of dopamine-transporter uptake on the right ipsilateral side of tremor in a patient with Parkinson’s disease [abstract]. Mov Disord 30:996
Kaasinen V (2015) Ipsilateral deficits of dopaminergic neurotransmission in Parkinson’s disease. Ann Clin Transl Neurol 3:21–26. https://doi.org/10.1002/acn3.268
Erro R, Barone P, Vicidomini C et al (2013) Patients with Parkinson’s disease and scans with (predominant) ipsilateral dopaminergic deficit. J Neurol 260:2405–2406. https://doi.org/10.1007/s00415-013-7030-z
Tazoe T, Perez MA (2014) Selective activation of ipsilateral motor pathways in intact humans. J Neurosci 34:13924–13934. https://doi.org/10.1523/JNEUROSCI.1648-14.2014
Tabbal SD, Ushe M, Mink JW et al (2008) Unilateral subthalamic nucleus stimulation has a measurable ipsilateral effect on rigidity and bradykinesia in Parkinson disease. Exp Neurol 211:234–242. https://doi.org/10.1016/j.expneurol.2008.01.024
Vitek JL, Bakay RAE, Freeman A et al (2003) Randomized trial of pallidotomy versus medical therapy for Parkinson’s disease. Ann Neurol 53:558–569. https://doi.org/10.1002/ana.10517
Kojovic M, Bologna M, Kassavetis P et al (2012) Functional reorganization of sensorimotor cortex in early Parkinson disease. Neurology 78:1441–1448. https://doi.org/10.1212/WNL.0b013e318253d5dd
Janicek AK, Avery RJ, Kuo PH (2014) The pinwheel sign: artifact from head rotation during SPECT acquisition for dopamine transporter imaging. J Nucl Med Technol 42:75–76. https://doi.org/10.2967/jnmt.113.126078
Covington MF, McMillan NA, Avery RJ, Kuo PH (2013) The semicolon sign: dopamine transporter imaging artifact from head tilt. J Nucl Med Technol 41:105–107. https://doi.org/10.2967/jnmt.112.117184
Erro R, Pappatà S, Picillo M et al (2013) Teaching neuroimages: pseudo-abnormal DaTscan findings in meningioma-induced parkinsonism. Neurology 80:e147–e147. https://doi.org/10.1212/WNL.0b013e318289709d
Booij J, Kemp P (2008) Dopamine transporter imaging with [(123)I]FP-CIT SPECT: potential effects of drugs. Eur J Nucl Med Mol Imaging 35:424–438. https://doi.org/10.1007/s00259-007-0621-0
Funding
This research did not receive any funding.
Author information
Authors and Affiliations
Contributions
RB: review of literature, interpretation of data, drafting of manuscript. PB: interpretation of data and critical revision of the manuscript. MF: interpretation of data and critical revision of the manuscript. RE: conception and design, interpretation of data, and critical revision of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
RB declares no conflict of interest. PB received consultancies as a member of the advisory board for Zambon, Lundbeck, UCB, Chiesi, Abbvie, and Acorda. FM is Editor-in-Chief of the Journal of Neurology and Associate Editor of Radiology, Human Brain Mapping and Neurological Sciences, received compensation for consulting services and/or speaking activities from Almiral, Alexion, Bayer, Biogen, Celgene, Eli Lilly, Genzyme, Merck-Serono, Novartis, Roche, Sanofi, Takeda, and Teva Pharmaceutical Industries, and receives research support from Biogen Idec, Merck-Serono, Novartis and Roche. RE received royalties from the publication of “Paroxysmal Movement Disorders” (Springer, 2020) and honoraria for speaking from the Movement Disorder Society.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Rights and permissions
About this article
Cite this article
Roberta, B., Paolo, B., Massimo, F. et al. Unexpected (123I)FP-CIT SPECT findings: SWIDD, SWEDD and all DAT. J Neurol 269, 758–770 (2022). https://doi.org/10.1007/s00415-021-10809-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10809-x