Abstract
Background
Advanced structural analyses are increasingly being highly valued to uncover pathophysiological understanding of anti-N-methyl-d-aspartate receptor (NMDAR) encephalitis. Therefore, we aimed to explore whether and how antibody-mediated NMDAR dysfunction affected cortical and sub-cortical brain morphology and their relationship with clinical symptoms.
Methods
We performed surface-based morphometry analyses, hippocampal segmentation, and correlational analyses in 24 patients with anti-NMDAR encephalitis after acute disease stage and 30 normal controls (NC) in this case–control study.
Results
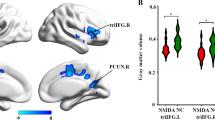
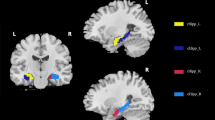
Patients showed significantly decreased cortical alterations mainly in language network (LN) and default mode network (DMN), as well as decreased gray matter volume in left cornu ammonis 1 (CA1) body of hippocampus. Further correlation analyses showed that the decreased cortical thickness in the right superior frontier gyrus was associated with decreased cognitive scores, the decreased cortical volume in the right pars triangulari and decreased surface area in the right pars operculari were associated with decreased memory scores, whereas decreased gray matter volume in the left CA1 body was significantly correlated with longer time between first symptom and imaging in the patients.
Conclusion
These results suggested that cognitive impairments resulted from long-term sequelae of the encephalitis were mainly associated with cortical alterations in LN and DMN and sub-cortical atrophy of left CA1 body, which can be served as effective features to assess disease progression in clinical routine examination.




Similar content being viewed by others
Data availability statement
The data used in this study are in-house data and can be available upon reasonable request from the corresponding author.
References
Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T et al (2016) A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 15(4):391–404
Deakin J, Lennox BR, Zandi MS (2014) Antibodies to the N-methyl-d-aspartate receptor and other synaptic proteins in psychosis. Biol Psychiatry 75(4):284–291
de Bruijn M, Aarsen FK, van Oosterhout MP, van der Knoop MM, Catsman-Berrevoets CE, Schreurs MWJ et al (2018) Long-term neuropsychological outcome following pediatric anti-NMDAR encephalitis. Neurology 90(22):e1997–e2005
McKeon GL, Robinson GA, Ryan AE, Blum S, Gillis D, Finke C et al (2018) Cognitive outcomes following anti-N-methyl-d-aspartate receptor encephalitis: a systematic review. J Clin Exp Neuropsychol 40(3):234–252
Zhang T, Duan Y, Ye J, Xu W, Shu N, Wang C et al (2018) Brain MRI characteristics of patients with anti-N-methyl-d-aspartate receptor encephalitis and their associations with 2-year clinical outcome. AJNR Am J Neuroradiol 39(5):824–829
Wang R, Lai XH, Liu X, Li YJ, Chen C, Li C et al (2018) Brain magnetic resonance-imaging findings of anti-N-methyl-d-aspartate receptor encephalitis: a cohort follow-up study in Chinese patients. J Neurol 265(2):362–369
Bacchi S, Franke K, Wewegama D, Needham E, Patel S, Menon D (2018) Magnetic resonance imaging and positron emission tomography in anti-NMDA receptor encephalitis: a systematic review. J Clin Neurosci 52:54–59
Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M et al (2008) Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 7(12):1091–1098
Titulaer MJ, McCracken L, Gabilondo I, Armangue T, Glaser C, Iizuka T et al (2013) Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 12(2):157–165
Heine J, Pruss H, Bartsch T, Ploner CJ, Paul F, Finke C (2015) Imaging of autoimmune encephalitis–Relevance for clinical practice and hippocampal function. Neuroscience 309:68–83
Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R (2011) Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 10(1):63–74
Thomas A, Rauschkolb P, Gresa-Arribas N, Schned A, Dalmau JO, Fadul CE (2013) Anti-N-methyl-d-aspartate receptor encephalitis: a patient with refractory illness after 25 months of intensive immunotherapy. JAMA Neurol 70(12):1566–1568
Cai L, Liang Y, Huang H, Zhou X, Zheng J (2020) Cerebral functional activity and connectivity changes in anti-N-methyl-d-aspartate receptor encephalitis: A resting-state fMRI study. Neuroimage Clin. 25:102189
Finke C, Kopp UA, Scheel M, Pech LM, Soemmer C, Schlichting J et al (2013) Functional and structural brain changes in anti-N-methyl-d-aspartate receptor encephalitis. Ann Neurol 74(2):284–296
Peer M, Prüss H, Ben-Dayan I, Paul F, Arzy S, Finke C (2017) Functional connectivity of large-scale brain networks in patients with anti-NMDA receptor encephalitis: an observational study. Lancet Psychiatry 4(10):768–774
Finke C, Kopp UA, Pajkert A, Behrens JR, Leypoldt F, Wuerfel JT et al (2016) Structural hippocampal damage following anti-N-methyl-d-aspartate receptor encephalitis. Biol Psychiatry 79(9):727–734
Palomero-Gallagher N, Amunts K, Zilles K (2015) Transmitter receptor distribution in the human brain. In: Toga AW (ed) Brain mapping. Academic Press, Waltham, pp 261–275
Brier MR, Day GS, Snyder AZ, Tanenbaum AB, Ances BM (2016) N-methyl-d-aspartate receptor encephalitis mediates loss of intrinsic activity measured by functional MRI. J Neurol 263(6):1083–1091
Miao A, Liu Q, Li Z, Liu W, Wang L, Ge J et al (2020) Altered cerebral blood flow in patients with anti-NMDAR encephalitis. J Neurol 267(6):1760–1773
Liang Y, Cai L, Zhou X, Huang H, Zheng J (2020) Voxel-based analysis and multivariate pattern analysis of diffusion tensor imaging study in anti-NMDA receptor encephalitis. Neuroradiology 62(2):231–239
Phillips OR, Joshi SH, Narr KL, Shattuck DW, Singh M, Di Paola M et al (2018) Superficial white matter damage in anti-NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry 89(5):518–525
Laurikainen H, Isotupa I, Nyman M, Ilonen T, Nummelin T, Salokangas RKR et al (2019) Longitudinal brain morphology in anti-NMDA receptor encephalitis: a case report with controls. BMC Psychiatry 19(1):145
Lee T, Yuen K, Chan C (2002) Normative data for neuropsychological measures of fluency, attention, and memory measures for Hong Kong Chinese. J Clin Exp Neuropsychol 24(5):615–632
Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M et al (2015) A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage 115:117–137
John CM, Mathew DE, Abdelaziz M, Mahmoud AAH, AlOtaibi AD, Sohal APS (2019) Anti-N-methyl-d-aspartate receptor encephalitis: a case series and review of the literature. J Pediatr Neurosci 14(4):180–185
McKeon G, Parker S, Warren N, Scott JG (2020) The patient experience of recovery following anti-NMDA receptor encephalitis: a qualitative content analysis. J Neuropsychiatry Clin Neurosci 2021;33(1):57–63.
Liu X, Zhang L, Chen C, Gong X, Lin J, An D et al (2019) Long-term cognitive and neuropsychiatric outcomes in patients with anti-NMDAR encephalitis. Acta Neurol Scand 140(6):414–421
Geschwind DH, Rakic P (2013) Cortical evolution: judge the brain by its cover. Neuron 80(3):633–647
Kremen WS, Fennema-Notestine C, Eyler LT, Panizzon MS, Chen CH, Franz CE et al (2013) Genetics of brain structure: contributions from the Vietnam Era Twin Study of Aging. Am J Med Genet B Neuropsychiatr Genet 162B(7):751–761
Chenn A, Walsh CA (2002) Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297(5580):365–369
Xu J, Wang J, Bai T, Zhang X, Li T, Hu Q et al (2019) Electroconvulsive therapy induces cortical morphological alterations in major depressive disorder revealed with surface-based morphometry analysis. Int J Neural Syst 29(7):1950005
Guo YY, Lu XY, Wu Y, Chen Y, Wei Q, Zhou N et al (2020) Cognitive function and cerebral blood perfusion changes in patients with anti-N-methyl-d-aspartate receptor encephalitis. Zhonghua Yi Xue Za Zhi 100(25):1942–1946
Catani M, Dell’acqua F, Vergani F, Malik F, Hodge H, Roy P et al (2012) Short frontal lobe connections of the human brain. Cortex 48(2):273–291
Ookawa S, Enatsu R, Kanno A, Ochi S, Akiyama Y, Kobayashi T et al (2017) Frontal fibers connecting the superior frontal gyrus to Broca area: a corticocortical evoked potential study. World Neurosurg 107:239–248
Fujii M, Maesawa S, Motomura K, Futamura M, Hayashi Y, Koba I et al (2015) Intraoperative subcortical mapping of a language-associated deep frontal tract connecting the superior frontal gyrus to Broca’s area in the dominant hemisphere of patients with glioma. J Neurosurg 122(6):1390–1396
Ursitti F, Roberto D, Papetti L, Moavero R, Ferilli MAN, Fusco L et al (2020) Diagnosis of pediatric anti-NMDAR encephalitis at the onset: a clinical challenge. Eur J Paediatr Neurol 30:9–16
Li R, Utevsky AV, Huettel SA, Braams BR, Peters S, Crone EA et al (2019) Developmental maturation of the precuneus as a functional core of the default mode network. J Cogn Neurosci 31(10):1506–1519
Zhong X, Pu W, Yao S (2016) Functional alterations of fronto-limbic circuit and default mode network systems in first-episode, drug-naive patients with major depressive disorder: a meta-analysis of resting-state fMRI data. J Affect Disord 206:280–286
Heine J, Pruss H, Kopp UA, Wegner F, Then Bergh F, Munte T et al (2018) Beyond the limbic system: disruption and functional compensation of large-scale brain networks in patients with anti-LGI1 encephalitis. J Neurol Neurosurg Psychiatry 89(11):1191–1199
Chen X, Huang L, Ye Q, Yang D, Qin R, Luo C et al (2019) Disrupted functional and structural connectivity within default mode network contribute to WMH-related cognitive impairment. Neuroimage Clin. 24:102088
Miraglia F, Vecchio F, Marra C, Quaranta D, Alu F, Peroni B et al (2020) Small world index in default mode network predicts progression from mild cognitive impairment to dementia. Int J Neural Syst 30(2):2050004
Qin Q, Tang Y, Dou X, Qu Y, Xing Y, Yang J et al (2020) Default mode network integrity changes contribute to cognitive deficits in subcortical vascular cognitive impairment, no dementia. Brain Imaging Behav 15:255–265
Wang C, Pan Y, Liu Y, Xu K, Hao L, Huang F et al (2018) Aberrant default mode network in amnestic mild cognitive impairment: a meta-analysis of independent component analysis studies. Neurol Sci 39(5):919–931
de Flores R, La Joie R, Chetelat G (2015) Structural imaging of hippocampal subfields in healthy aging and Alzheimer’s disease. Neuroscience 309:29–50
Mak E, Gabel S, Su L, Williams GB, Arnold R, Passamonti L et al (2017) Multi-modal MRI investigation of volumetric and microstructural changes in the hippocampus and its subfields in mild cognitive impairment, Alzheimer’s disease, and dementia with Lewy bodies. Int Psychogeriatr 29(4):545–555
La C, Linortner P, Bernstein JD, Ua Cruadhlaoich MAI, Fenesy M, Deutsch GK et al (2019) Hippocampal CA1 subfield predicts episodic memory impairment in Parkinson’s disease. Neuroimage Clin. 23:101824
Ding SL, Van Hoesen GW (2015) Organization and detailed parcellation of human hippocampal head and body regions based on a combined analysis of cyto- and chemoarchitecture. J Comp Neurol 523(15):2233–2253
Bello-Medina PC, Prado-Alcala RA, Rivas-Arancibia S (2019) Effect of ozone exposure on dendritic spines of CA1 pyramidal neurons of the dorsal hippocampus and on object-place recognition memory in rats. Neuroscience 402:1–10
Kesner RP, Kirk RA, Yu Z, Polansky C, Musso ND (2016) Dentate gyrus supports slope recognition memory, shades of grey-context pattern separation and recognition memory, and CA3 supports pattern completion for object memory. Neurobiol Learn Mem 129:29–37
Knierim JJ, Neunuebel JP (2016) Tracking the flow of hippocampal computation: pattern separation, pattern completion, and attractor dynamics. Neurobiol Learn Mem 129:38–49
Funding
This work was supported by funding from the National Natural Science Foundation of China (62006220), the Science Fund for Distinguished Young Scholars of Anhui Province (1808085J23), and Shenzhen Science and Technology Research Program (JCYJ20200109114816594).
Author information
Authors and Affiliations
Contributions
QH, YT, and KW designed the study. YG, XL, and JZ collected the data. JX, JZ, and JL analyzed the data. JX and JL wrote the manuscript. All authors discussed the results and reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
All authors have no competing interests to declare.
Ethical standard statement
The study was approved by the ethical committee of Anhui Medical University, and written informed consents were obtained from all participants according to the Declaration of Helsinki.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, J., Guo, Y., Li, J. et al. Progressive cortical and sub-cortical alterations in patients with anti-N-methyl-d-aspartate receptor encephalitis. J Neurol 269, 389–398 (2022). https://doi.org/10.1007/s00415-021-10643-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10643-1