Abstract
Objective
To characterize the clinical and pathological features of anti-HMGCR myopathy.
Methods
The presence of anti-HMGCR antibody in the serum of 227 patients with idiopathic inflammatory myopathy (IIM) and 100 healthy control individuals was assessed by ELISA. All ELISA positive samples were retested by indirect immunofluorescence assay (IIFA) on HEK293 cells. The clinical findings, muscle pathological features, and treatment outcomes of patients with anti-HMGCR myopathy, along with comparisons between anti-HMGCR myopathy with and without dermatomyositis (DM)-like skin rashes, and among MSA-based subgroups were analyzed.
Results
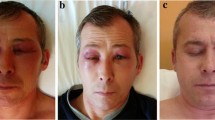
We established an optimized ELISA cutoff for anti-HMGCR antibody positivity as ≥ 5.28 U. The overall concordance between ELISA and IIFA was 96.83%. Twenty-one out of 227 IIM patients were anti-HMGCR-positive by both assays. Of these 21 patients, 9 had DM-like skin rashes, and 16 showed remarkable muscle inflammation; 5 patients were juvenile-onset, and 2 received statin treatment. The muscle biopsies from these patients demonstrated variable muscle necrosis and T cell infiltration. Most anti-HMGCR-positive patients achieved favorable outcomes following prednisone and additional immunotherapies. The anti-HMGCR myopathy patients with DM-like rashes, compared to those without DM-like rashes, were younger and had a shorter disease duration.
Conclusions
Optimization of cutoff of anti-HMGCR antibody assays with confirmation by alternative assays can result in higher sensitivity and specificity. DM-like skin rashes and lymphocytic infiltrates were not rare in patients with anti-HMGCR myopathy. These findings suggest that while anti-HMGCR myopathy may overlap with DM-like rash, it is pathologically different from classic DM, and should be considered a distinct subgroup of IIM.




Similar content being viewed by others
Data availability
All data relevant to the study are either included in the article or will be shared at the request of any qualified investigator.
References
Musset L, Allenbach Y, Benveniste O, Boyer O, Bossuyt X, Bentow C, Phillips J, Mammen A, Van Damme P, Westhovens R, Ghirardello A, Doria A, Choi MY, Fritzler MJ, Schmeling H, Muro Y, Garcia-De La Torre I, Ortiz-Villalvazo MA, Bizzaro N, Infantino M, Imbastaro T, Peng Q, Wang G, Vencovsky J, Klein M, Krystufkova O, Franceschini F, Fredi M, Hue S, Belmondo T, Danko K, Mahler M (2016) Anti-HMGCR antibodies as a biomarker for immune-mediated necrotizing myopathies: a history of statins and experience from a large international multi-center study. Autoimmun Rev 15(10):983–993. https://doi.org/10.1016/j.autrev.2016.07.023
Mammen AL, Chung T, Christopher-Stine L, Rosen P, Rosen A, Doering KR, Casciola-Rosen LA (2011) Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 63(3):713–721. https://doi.org/10.1002/art.30156
Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL (2010) A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum 62(9):2757–2766. https://doi.org/10.1002/art.27572
Watanabe Y, Suzuki S, Nishimura H, Murata KY, Kurashige T, Ikawa M, Asahi M, Konishi H, Mitsuma S, Kawabata S, Suzuki N, Nishino I (2015) Statins and myotoxic effects associated with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase autoantibodies: an observational study in Japan. Medicine (Baltimore). https://doi.org/10.1097/MD.0000000000000416
Allenbach Y, Benveniste O, Goebel HH, Stenzel W (2017) Integrated classification of inflammatory myopathies. Neuropathol Appl Neurobiol 43(1):62–81. https://doi.org/10.1111/nan.12380
Ghirardello A, Doria A (2018) New insights in myositis-specific autoantibodies. Curr Opin Rheumatol 30(6):614–622. https://doi.org/10.1097/BOR.0000000000000548
Mariampillai K, Granger B, Amelin D, Guiguet M, Hachulla E, Maurier F, Meyer A, Tohme A, Charuel JL, Musset L, Allenbach Y, Benveniste O (2018) Development of a new classification system for idiopathic inflammatory myopathies based on clinical manifestations and myositis-specific autoantibodies. JAMA Neurol 75(12):1528–1537. https://doi.org/10.1001/jamaneurol.2018.2598
Selva-O’Callaghan A, Pinal-Fernandez I, Trallero-Araguas E, Milisenda JC, Grau-Junyent JM, Mammen AL (2018) Classification and management of adult inflammatory myopathies. Lancet Neurol 17(9):816–828. https://doi.org/10.1016/S1474-4422(18)30254-0
Pinal-Fernandez I, Casal-Dominguez M, Mammen AL (2018) Immune-mediated necrotizing myopathy. Curr Rheumatol Rep 20(4):21. https://doi.org/10.1007/s11926-018-0732-6
Hoogendijk JE, Amato AA, Lecky BR, Choy EH, Lundberg IE, Rose MR, Vencovsky J, de Visser M, Hughes RA (2004) 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10–12 October 2003, Naarden The Netherlands. Neuromuscul Disord 14(5):337–345. https://doi.org/10.1016/j.nmd.2004.02.006
Allenbach Y, Mammen AL, Benveniste O, Stenzel W, Working I-M, G, (2018) 224th ENMC International Workshop: clinico-sero-pathological classification of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14–16 October 2016. Neuromuscul Disord 28(1):87–99. https://doi.org/10.1016/j.nmd.2017.09.016
Limaye V, Bundell C, Hollingsworth P, Rojana-Udomsart A, Mastaglia F, Blumbergs P, Lester S (2015) Clinical and genetic associations of autoantibodies to 3-hydroxy-3-methyl-glutaryl-coenzyme a reductase in patients with immune-mediated myositis and necrotizing myopathy. Muscle Nerve 52(2):196–203. https://doi.org/10.1002/mus.24541
Hida A, Yamashita T, Hosono Y, Inoue M, Kaida K, Kadoya M, Miwa Y, Yajima N, Maezawa R, Arai S, Kurasawa K, Ito K, Shimada H, Iwanami T, Sonoo M, Hatanaka Y, Murayama S, Uchibori A, Chiba A, Aizawa H, Momoo T, Nakae Y, Sakurai Y, Shiio Y, Hashida H, Yoshizawa T, Sakiyama Y, Oda A, Inoue K, Takeuchi S, Iwata NK, Date H, Masuda N, Mikata T, Motoyoshi Y, Uesaka Y, Maeda MH, Nakashima R, Tsuji S, Kwak S, Mimori T, Shimizu J (2016) Anti-TIF1-gamma antibody and cancer-associated myositis: a clinicohistopathologic study. Neurology 87(3):299–308. https://doi.org/10.1212/WNL.0000000000002863
Watanabe Y, Uruha A, Suzuki S, Nakahara J, Hamanaka K, Takayama K, Suzuki N, Nishino I (2016) Clinical features and prognosis in anti-SRP and anti-HMGCR necrotising myopathy. J Neurol Neurosurg Psychiatry 87(10):1038–1044. https://doi.org/10.1136/jnnp-2016-313166
Ge Y, Lu X, Peng Q, Shu X, Wang G (2015) Clinical characteristics of anti-3-hydroxy-3-methylglutaryl coenzyme a reductase antibodies in Chinese patients with idiopathic inflammatory myopathies. PLoS ONE. https://doi.org/10.1371/journal.pone.0141616
Jiao Y, Cai S, Lin J, Zhu W, Xi J, Li J, Yue D, Zhang T, Qiao K, Wang Y, Zhao C, Lu J (2018) Statin-naive anti-HMGCR antibody-mediated necrotizing myopathy in China. J Clin Neurosci 57:13–19. https://doi.org/10.1016/j.jocn.2018.08.010
Alshehri A, Choksi R, Bucelli R, Pestronk A (2015) Myopathy with anti-HMGCR antibodies: perimysium and myofiber pathology. Neurol Neuroimmunol Neuroinflamm. https://doi.org/10.1212/NXI.0000000000000124
Kadoya M, Hida A, Hashimoto Maeda M, Taira K, Ikenaga C, Uchio N, Kubota A, Kaida K, Miwa Y, Kurasawa K, Shimada H, Sonoo M, Chiba A, Shiio Y, Uesaka Y, Sakurai Y, Izumi T, Inoue M, Kwak S, Tsuji S, Shimizu J (2016) Cancer association as a risk factor for anti-HMGCR antibody-positive myopathy. Neurol Neuroimmunol Neuroinflamm. https://doi.org/10.1212/NXI.0000000000000290
Chung T, Christopher-Stine L, Paik JJ, Corse A, Mammen AL (2015) The composition of cellular infiltrates in anti-HMG-CoA reductase-associated myopathy. Muscle Nerve 52(2):189–195. https://doi.org/10.1002/mus.24642
Aggarwal R, Moghadam-Kia S, Lacomis D, Malik A, Qi Z, Koontz D, Burlingame RW, Oddis CV (2019) Anti-hydroxy-3-methylglutaryl-coenzyme A reductase (anti-HMGCR) antibody in necrotizing myopathy: treatment outcomes, cancer risk, and role of autoantibody level. Scand J Rheumatol. https://doi.org/10.1080/03009742.2019.1672782
Musset L, Miyara M, Benveniste O, Charuel JL, Shikhman A, Boyer O, Fowler R, Mammen A, Phillips J, Mahler M (2014) Analysis of autoantibodies to 3-hydroxy-3-methylglutaryl-coenzyme A reductase using different technologies. J Immunol Res. https://doi.org/10.1155/2014/405956
Kassardjian CD, Lennon VA, Alfugham NB, Mahler M, Milone M (2015) Clinical features and treatment outcomes of necrotizing autoimmune myopathy. JAMA Neurol 72(9):996–1003. https://doi.org/10.1001/jamaneurol.2015.1207
Drouot L, Allenbach Y, Jouen F, Charuel JL, Martinet J, Meyer A, Hinschberger O, Bader-Meunier B, Kone-Paut I, Campana-Salort E, Eymard B, Tournadre A, Musset L, Sibilia J, Marie I, Benveniste O, Boyer O, French Myositis N (2014) Exploring necrotizing autoimmune myopathies with a novel immunoassay for anti-3-hydroxy-3-methyl-glutaryl-CoA reductase autoantibodies. Arthritis Res Ther 16(1):R39. https://doi.org/10.1186/ar4468
Alvarado-Cardenas M, Marin-Sanchez A, Martinez MA, Martinez-Martinez L, Pinal-Fernandez I, Labrador-Horrillo M, Balada E, Mundet-Tuduri X, Gonzalez-Mera L, Casademont J, Acebes EM, Moreno PJ, Juarez C, Grau-Junyent JM, Pujol-Borrell R, Selva-O’Callaghan A (2016) Statin-associated autoimmune myopathy: a distinct new IFL pattern can increase the rate of HMGCR antibody detection by clinical laboratories. Autoimmun Rev 15(12):1161–1166. https://doi.org/10.1016/j.autrev.2016.09.005
Lundberg IE, Tjarnlund A, Bottai M, Werth VP, Pilkington C, Visser M, Alfredsson L, Amato AA, Barohn RJ, Liang MH, Singh JA, Aggarwal R, Arnardottir S, Chinoy H, Cooper RG, Danko K, Dimachkie MM, Feldman BM, Torre IG, Gordon P, Hayashi T, Katz JD, Kohsaka H, Lachenbruch PA, Lang BA, Li Y, Oddis CV, Olesinska M, Reed AM, Rutkowska-Sak L, Sanner H, Selva-O’Callaghan A, Song YW, Vencovsky J, Ytterberg SR, Miller FW, Rider LG, International Myositis Classification Criteria Project consortium TEr, The Juvenile Dermatomyositis Cohort Biomarker S, Repository (2017) 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis 76(12):1955–1964. https://doi.org/10.1136/annrheumdis-2017-211468
Mammen AL, Allenbach Y, Stenzel W, Benveniste O, Group EtWS (2020) 239th ENMC International Workshop: classification of dermatomyositis, Amsterdam, the Netherlands, 14–16 December 2018. Neuromuscul Disord 30(1):70–92. https://doi.org/10.1016/j.nmd.2019.10.005
Suzuki S, Yonekawa T, Kuwana M, Hayashi YK, Okazaki Y, Kawaguchi Y, Suzuki N, Nishino I (2014) Clinical and histological findings associated with autoantibodies detected by RNA immunoprecipitation in inflammatory myopathies. J Neuroimmunol 274(1–2):202–208. https://doi.org/10.1016/j.jneuroim.2014.07.006
Liu M, Hou Y, Dai T, Lv J, Li W, Zhao Y, Fang Q, Yan C (2019) The clinical and histopathological features of idiopathic inflammatory myopathies with asymmetric muscle involvement. J Clin Neurosci 65:46–53. https://doi.org/10.1016/j.jocn.2019.04.002
Allenbach Y, Keraen J, Bouvier AM, Jooste V, Champtiaux N, Hervier B, Schoindre Y, Rigolet A, Gilardin L, Musset L, Charuel JL, Boyer O, Jouen F, Drouot L, Martinet J, Stojkovic T, Eymard B, Laforet P, Behin A, Salort-Campana E, Fain O, Meyer A, Schleinitz N, Mariampillai K, Grados A, Benveniste O (2016) High risk of cancer in autoimmune necrotizing myopathies: usefulness of myositis specific antibody. Brain 139(Pt 8):2131–2135. https://doi.org/10.1093/brain/aww054
Tiniakou E, Pinal-Fernandez I, Lloyd TE, Albayda J, Paik J, Werner JL, Parks CA, Casciola-Rosen L, Christopher-Stine L, Mammen AL (2017) More severe disease and slower recovery in younger patients with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Rheumatology (Oxford) 56(5):787–794. https://doi.org/10.1093/rheumatology/kew470
Waters PJ, McKeon A, Leite MI, Rajasekharan S, Lennon VA, Villalobos A, Palace J, Mandrekar JN, Vincent A, Bar-Or A, Pittock SJ (2012) Serologic diagnosis of NMO: a multicenter comparison of aquaporin-4-IgG assays. Neurology 78(9):665–671. https://doi.org/10.1212/WNL.0b013e318248dec1 (discussion 669)
Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, Dessain SK, Rosenfeld MR, Balice-Gordon R, Lynch DR (2008) Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 7(12):1091–1098. https://doi.org/10.1016/S1474-4422(08)70224-2
Hou Y, Luo YB, Dai T, Shao K, Li W, Zhao Y, Lu JQ, Yan C (2018) Revisiting pathological classification criteria for adult idiopathic inflammatory myopathies: in-depth analysis of muscle biopsies and correlation between pathological diagnosis and clinical manifestations. J Neuropathol Exp Neurol 77(5):395–404. https://doi.org/10.1093/jnen/nly017
Klein M, Mann H, Plestilova L, Zamecnik J, Betteridge Z, McHugh N, Vencovsky J (2015) Increasing incidence of immune-mediated necrotizing myopathy: single-centre experience. Rheumatology (Oxford) 54(11):2010–2014. https://doi.org/10.1093/rheumatology/kev229
Allenbach Y, Drouot L, Rigolet A, Charuel JL, Jouen F, Romero NB, Maisonobe T, Dubourg O, Behin A, Laforet P, Stojkovic T, Eymard B, Costedoat-Chalumeau N, Campana-Salort E, Tournadre A, Musset L, Bader-Meunier B, Kone-Paut I, Sibilia J, Servais L, Fain O, Larroche C, Diot E, Terrier B, De Paz R, Dossier A, Menard D, Morati C, Roux M, Ferrer X, Martinet J, Besnard S, Bellance R, Cacoub P, Arnaud L, Grosbois B, Herson S, Boyer O, Benveniste O, French Myositis N (2014) Anti-HMGCR autoantibodies in European patients with autoimmune necrotizing myopathies: inconstant exposure to statin. Medicine (Baltimore) 93(3):150–157. https://doi.org/10.1097/MD.0000000000000028
Alarcon J, Aguila S, Arancibia-Avila P, Fuentes O, Zamorano-Ponce E, Hernandez M (2003) Production and purification of statins from Pleurotus ostreatus (Basidiomycetes) strains. Z Naturforsch C J Biosci 58(1–2):62–64. https://doi.org/10.1515/znc-2003-1-211
Werner JL, Christopher-Stine L, Ghazarian SR, Pak KS, Kus JE, Daya NR, Lloyd TE, Mammen AL (2012) Antibody levels correlate with creatine kinase levels and strength in anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Rheum 64(12):4087–4093. https://doi.org/10.1002/art.34673
Kishi T, Rider LG, Pak K, Barillas-Arias L, Henrickson M, McCarthy PL, Shaham B, Weiss PF, Horkayne-Szakaly I, Targoff IN, Miller FW, Mammen AL, Childhood Myositis Heterogeneity Study G (2017) Association of Anti-3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase Autoantibodies With DRB1*07:01 and severe myositis in juvenile myositis patients. Arthritis Care Res (Hoboken) 69(7):1088–1094. https://doi.org/10.1002/acr.23113
Acknowledgements
The authors thank Inova Diagnostics for providing HMGCR ELISA kits and thank all the patients and healthy controls included in this study.
Funding
This work was supported by the Taishan Scholars Program of Shandong Province, the National Nature Science Foundation of China (No.81701238), 20 policy supported projects of collaborative innovation and achievement transformation in universities and research institutes of Jinan (2019GXRC050), Shandong Provincial Natural Science Foundation (Grant Number ZR2020QH167), Innovative Research Project of Undergraduate Clinical Medicine Teaching of Qilu Hospital, Shandong University (Grant Number 2019QLJY13), Qingdao Technology Program for Health and Welfare (20-3-4-42-nsh), Qingdao Applied Basic Research Source Innovation Plan (19-6-2-78-cg), and Qingdao Key Health Discipline Development Fund.
Author information
Authors and Affiliations
Contributions
YH and KS contributed equally to this work. CY designed and conceptualized study. YH and KS analyzed the data and drafted the manuscript. KS, YY, and GN tested antibodies. CY, YH, TD, WL, YZ, and DL, major role in the acquisition of data. CY, JL, and GN interpreted the data and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Qilu Hospital (Qingdao), Shandong University, China (KYLL-qdql2020019).
Informed consent
All patients or their guardian gave their written informed consent for participation in the study and further publications.
Rights and permissions
About this article
Cite this article
Hou, Y., Shao, K., Yan, Y. et al. Anti-HMGCR myopathy overlaps with dermatomyositis-like rash: a distinct subtype of idiopathic inflammatory myopathy. J Neurol 269, 280–293 (2022). https://doi.org/10.1007/s00415-021-10621-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10621-7