Abstract
Objectives
To clarify whether serum neurofilament light chains (NfLs) serve as a biomarker of axonal damage in patients with chronic inflammatory demyelinating polyneuropathy (CIDP), especially in patients with anti-neurofascin 155 (NF155) antibodies.
Methods
The Simoa system was used to examine serum NfL levels from 58 patients with CIDP, including 13 anti-NF155 antibody-positive patients, and from 14 age- and sex-matched healthy individuals. Serum NfL levels were evaluated before and after treatment in eight patients with anti-NF155 antibodies. Clinical features, electrophysiological findings, and cerebrospinal fluid (CSF) protein levels, were evaluated. The pathological features of sural nerves from 40 patients were also examined.
Results
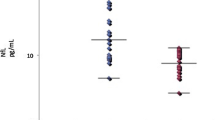
Serum NfL levels were significantly higher in patients with CIDP than in healthy individuals (median 29.63 vs. 7.71 pg/mL, p < 0.001) and were correlated with both modified Rankin Scale scores (r = 0.584, p < 0.001) and CSF protein levels (r = 0.432, p = 0.001). The NfL levels of anti-NF155 antibody-positive patients were higher than those of antibody-negative patients (p = 0.005). Serum NfL levels were negatively correlated with compound muscle action potential amplitudes of the tibial nerves (r = − 0.404, p = 0.004) and positively correlated with the degree of active axonal degeneration in the pathological findings (r = 0.485, p = 0.001). In the antibody-positive group, NfL levels and antibody titers decreased after treatment in all examined patients.
Conclusion
Serum NfL correlated with pathological indices of axonal degeneration, and may serve as a biomarker that reflects active axonal damage of CIDP.



Similar content being viewed by others
Data availability
The data that support the findings are available from the corresponding author upon reasonable request.
References
Dyck PJ, Lais AC, Ohta M et al (1975) Chronic inflammatory polyradiculoneuropathy. Mayo Clin Proc 50(11):621–637
Van den Bergh PY, Hadden RD, Bouche P et al (2010) European Federation of neurological societies/peripheral nerve society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint Task Force of the European Federation of Neurological societies and the peripheral nerve society – first revision. J Peripher Nerv Syst 15(3):185–195
Dalakas MC (2011) Advances in the diagnosis, pathogenesis and treatment of CIDP. Nat Rev Neurol 7(9):507–517
Latov N (2014) Diagnosis and treatment of chronic acquired demyelinating polyneuropathies. Nat Rev Neurol 10(8):435–446
Bunschoten C, Jacobs BC, Van den Bergh PYK et al (2019) Progress in diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy. Lancet Neurol 18(8):784–794
Koike H, Katsuno M (2020) Pathophysiology of chronic inflammatory demyelinating polyneuropathy: insights into classification and therapeutic strategy. Neurol Ther. https://doi.org/10.1007/s40120-020-00190-8
Ng JK, Malotka J, Kawakami N et al (2012) Neurofascin as a target for autoantibodies in peripheral neuropathies. Neurology 79(23):2241–2248
Querol L, Nogales-Gadea G, Rojas-Garcia R et al (2013) Antibodies to contactin-1 in chronic inflammatory demyelinating polyneuropathy. Ann Neurol 73(3):370–380
Querol L, Nogales-Gadea G, Rojas-Garcia R et al (2014) Neurofascin IgG4 antibodies in CIDP associate with disabling tremor and poor response to IVIg. Neurology 82(10):879–886
Doppler K, Appeltshauser L, Wilhelmi K et al (2015) Destruction of paranodal architecture in inflammatory neuropathy with anti-contactin-1 autoantibodies. J Neurol Neurosurg Psychiatry 86(7):720–728
Miura Y, Devaux JJ, Fukami Y et al (2015) Contactin 1 IgG4 associates to chronic inflammatory demyelinating polyneuropathy with sensory ataxia. Brain 138(6):1484–1491
Ogata H, Yamasaki R, Hiwatashi A et al (2015) Characterization of IgG4 anti-neurofascin 155 antibody-positive polyneuropathy. Ann Clin Transl Neurol 2(10):960–971
Devaux JJ, Miura Y, Fukami Y et al (2016) Neurofascin-155 IgG4 in chronic inflammatory demyelinating polyneuropathy. Neurology 86(9):800–807
Koike H, Kadoya M, Kaida KI et al (2017) Paranodal dissection in chronic inflammatory demyelinating polyneuropathy with anti-neurofascin-155 and anti-contactin-1 antibodies. J Neurol Neurosurg Psychiatry 88(6):465–473
Vallat JM, Yuki N, Sekiguchi K et al (2017) Paranodal lesions in chronic inflammatory demyelinating polyneuropathy associated with anti-Neurofascin 155 antibodies. Neuromuscul Disord 27(3):290–293
Koike H, Nishi R, Ikeda S et al (2018) Ultrastructural mechanisms of macrophage-induced demyelination in CIDP. Neurology 91(23):1051–1060
Iijima M, Yamamoto M, Hirayama M et al (2005) Clinical and electrophysiologic correlates of IVIg responsiveness in CIDP. Neurology 64(8):1471–1475
Rajabally YA (2015) Long-term immunoglobulin therapy for chronic inflammatory demyelinating polyradiculoneuropathy. Muscle Nerve 51(5):657–661
Notturno F, Capasso M, DeLauretis A et al (2009) Glial fibrillary acidic protein as a marker of axonal damage in chronic neuropathies. Muscle Nerve 40(1):50–54
Wong AH, Fukami Y, Sudo M et al (2016) Sialylated IgG-Fc: a novel biomarker of chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry 87(3):275–279
Allen JA, Merkies ISJ, Lewis RA (2020) Monitoring clinical course and treatment response in chronic inflammatory demyelinating polyneuropathy during routine care: a review of clinical and laboratory assessment measures. JAMA Neurol 77(9):1159–1166
Lu CH, Macdonald-Wallis C, Gray E et al (2015) Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology 84(22):2247–2257
Steinacker P, Feneberg E, Weishaupt J et al (2016) Neurofilaments in the diagnosis of motoneuron diseases: a prospective study on 455 patients. J Neurol Neurosurg Psychiatry 87(1):12–20
Preische O, Schultz SA, Apel A et al (2019) Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer’s disease. Nat Med 25(2):277–283
Mattsson N, Andreasson U, Zetterberg H et al (2017) Alzheimer’s disease neuroimaging initiative. Association of plasma neurofilament light with neurodegeneration in with alzheimer disease. JAMA Neurol 74(5):557–566
Lycke JN, Karlsson JE, Andersen O et al (1998) Neurofilament protein in cerebrospinal fluid: a potential marker of activity in multiple sclerosis. J Neurol Neurosurg Psychiatry 64(3):402–404
Malmeström C, Haghighi S, Rosengren L et al (2003) Neurofilament light protein and glial fibrillary acidic protein as biological markers in MS. Neurology 61(12):1720–1725
Gunnarsson M, Malmeström C, Axelsson M et al (2011) Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann Neurol 69(1):83–89
Kuhle J, Kropshofer H, Haering DA et al (2019) Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 92(10):e1007–e1015
Sandelius Å, Zetterberg H, Blennow K et al (2018) Plasma neurofilament light chain concentration in the inherited peripheral neuropathies. Neurology 90(6):e518–e524
Mariotto S, Farinazzo A, Magliozzi R et al (2018) Serum and cerebrospinal neurofilament light chain levels in patients with acquired peripheral neuropathies. J Peripher Nerv Syst 23(3):174–177
van Lieverloo GGA, Wieske L, Verhamme C et al (2019) Serum neurofilament light chain in chronic inflammatory demyelinating polyneuropathy. J Peripher Nerv Syst 24(2):187–194
Ikeda S, Koike H, Nishi R et al (2019) Clinicopathological characteristics of subtypes of chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol Neurosurg Psychiatry 90(9):988–996
Dyck PJ, Dyck PJB, Engelstad J (2005) Pathologic alterations of nerves. In: Dyck PJ, Thomas PK (eds) Peripheral neuropathy, 4th edn. Elsevier, pp 733–829
Gafson AR, Barthélemy NR, Bomont P et al (2020) Neurofilaments: neurobiological foundations for biomarker applications. Brain 143(7):1975–1998
Lombardi V, Querin G, Ziff OJ et al (2019) Muscle and not neuronal biomarkers correlate with severity in spinal and bulbar muscular atrophy. Neurology 92(11):e1205–e1211
Kapoor M, Foiani M, Heslegrave A et al (2019) Plasma neurofilament light chain concentration is increased and correlates with the severity of neuropathy in hereditary transthyretin amyloidosis. J Peripher Nerv Syst 24(4):314–319
Shimizu F, Sawai S, Sano Y et al (2014) Severity and patterns of blood-nerve barrier breakdown in patients with chronic inflammatory demyelinating polyradiculoneuropathy: correlations with clinical subtypes. PLoS ONE 9(8):e104205
Mariotto S, Carta S, Bozzetti S et al (2020) Sural nerve biopsy: current role and comparison with serum neurofilament light chain levels. J Neurol 267(10):2881–2887
Fujita A, Ogata H, Yamasaki R et al (2018) Parallel fluctuation of anti-neurofascin 155 antibody levels with clinico-electrophysiological findings in patients with chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol Sci 384:107–112
Shimizu S, Iijima M, Fukami Y et al (2020) Efficacy and safety of rituximab in refractory CIDP with or without IgG4 autoantibodies (RECIPE): protocol for a double-blind, randomized Placebo-Controlled Clinical Trial. JMIR Res Protoc 9(4):e17117
Acknowledgements
We thank Bronwen Gardner, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/) for editing a draft of this manuscript.
Funding
Dr. Fukami, Dr. Koike, Dr. Yamada and Dr. Hashizume report no disclosures. Dr. Iijima is supported by grants by JSPS KAKENHI (JP20K07864), the Japan Agency for Medical Research and Development (20lk0201080), Zenyaku Kogyo Co. Ltd., and Japan Blood Products Organization (JBPO). He also received honoraria from CSL Behring Co. Ltd. Dr. Katsuno is supported by a JSPS KAKENHI Grant Number JP20H00527, grants from the Japan Agency for Medical Research and Development (Nos. 19ek0109221, 19ek0109359, 19dk0207027, 19lk0201101, and 19dm0107155), a grant from the Naito Foundation and a grant from the Hori Sciences and Arts Foundation. He received honoraria from Takeda Pharmaceutical Co. Ltd., Alnylam Japan, Daiichi Sankyo Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Novartis Pharma Co. Ltd., Biogen Japan and UCB Japan and grants from Zenyaku Kogyo Co. Ltd., Japan Blood Products Organization, Mitsubishi-Tanabe Pharma, CSL Behring Co. Ltd., Dainippon Sumitomo Pharma Co. Ltd., Otsuka Pharmaceutical Co. Ltd. and Daiichi Sankyo Co. Ltd.
Author information
Authors and Affiliations
Contributions
Drafting/revising the manuscript for content: YF, MI, HK, and MK. Study concept and design: YF, MI, and MK. Acquisition of samples, data, analysis and interpretation of data: YF, MI, HK, SY, AH and MK.
Corresponding authors
Ethics declarations
Conflict of interest
None.
Ethics approval
Ethics Review Committee of the Nagoya University Graduate School of Medicine, Nagoya, Japan.
Provenance and peer review
Not commissioned; externally peer reviewed.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fukami, Y., Iijima, M., Koike, H. et al. Association of serum neurofilament light chain levels with clinicopathology of chronic inflammatory demyelinating polyneuropathy, including NF155 reactive patients. J Neurol 268, 3835–3844 (2021). https://doi.org/10.1007/s00415-021-10537-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10537-2