Abstract
Objective
We sought to test the hypothesis that technology could predict the risk of falls in Parkinson’s disease (PD) patients with orthostatic hypotension (OH) with greater accuracy than in-clinic assessment.
Methods
Twenty-six consecutive PD patients with OH underwent clinical (including home-like assessments of activities of daily living) and kinematic evaluations of balance and gait as well as beat-to-beat blood pressure (BP) monitoring to estimate their association with the risk of falls. Fall frequency was captured by a diary collected prospectively over 6 months. When applicable, the sensitivity, specificity, and diagnostic accuracy were measured using the area under the receiver operating characteristics curve (AUC). Additional in-clinic assessments included the OH Symptom Assessment (OHSA), the OH Daily Activity Score (OHDAS), and the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS).
Results
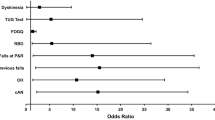
The prevalence of falls was 53.8% over six months. There was no association between the risk of falls and test of gait and postural stability (p ≥ 0.22) or home-like activities of daily living (p > 0.08). Conversely, kinematic data (waist sway during time-up-and-go, jerkiness, and centroidal frequency during postural sway with eyes-opened) predicted the risk of falls with high sensitivity and specificity (> 80%; AUC ≥ 0.81). There was a trend for higher risk of falls in patients with orthostatic mean arterial pressure ≤ 75 mmHg.
Conclusions
Kinematic but not clinical measures predicted falls in PD patients with OH. Orthostatic mean arterial pressure ≤ 75 mmHg may represent a hemodynamic threshold below which falls become more prevalent, supporting the aggressive deployment of corrective measures.

Similar content being viewed by others
References
Espay AJ, LeWitt PA, Hauser RA, Merola A, Masellis M, Lang AE (2016) Neurogenic orthostatic hypotension and supine hypertension in Parkinson's disease and related synucleinopathies: prioritisation of treatment targets. Lancet Neurol 15:954–966
LeWitt PA, Kymes S, Hauser RA (2020) Parkinson disease and orthostatic hypotension in the elderly: recognition and management of risk factors for falls. Aging Dis 11:679–691
Merola A, Sawyer RP, Artusi CAA, Suri R, Berndt Z, Lopez-Castellanos JR, Vaughan J, Vizcarra JA, Romagnolo A, Espay AJ (2018) Orthostatic hypotension in Parkinson disease: impact on health care utilization. Parkinsonism Relat Disord 47:45–49
Merola A, Romagnolo A, Rosso M, Lopez-Castellanos JR, Wissel BD, Larkin S, Bernardini A, Zibetti M, Maule S, Lopiano L, Espay AJ (2016) Orthostatic hypotension in Parkinson's disease: does it matter if asymptomatic? Parkinsonism Relat Disord 33:65–71
FitzGerald JJ, Lu Z, Jareonsettasin P, Antoniades CA (2018) Quantifying motor impairment in movement disorders. Front Neurosci 12:202
Zampogna A, Mileti I, Palermo E, Celletti C, Paoloni M, Manoni A, Mazzetta I, Dalla Costa G, Pérez-López C, Camerota F, Leocani L, Cabestany J, Irrera F, Suppa A (2020) Fifteen years of wireless sensors for balance assessment in neurological disorders. Sensors (Basel, Switzerland) 20:3247
Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry 51:745–752
Lahrmann H, Cortelli P, Hilz M, Mathias CJ, Struhal W, Tassinari M (2006) EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol 13:930–936
Dineen J, Freeman R (2015) Autonomic neuropathy. Semin Neurol 35:458–468
Tinetti ME, Williams TF, Mayewski R (1986) Fall risk index for elderly patients based on number of chronic disabilities. Am J Med 80:429–434
Kaufmann H, Malamut R, Norcliffe-Kaufmann L, Rosa K, Freeman R (2012) The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. Clin Auton Res 22:79–90
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N (2008) Movement Disorder Society UPDRS Revision Task Force, Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23:2129–2170
Goetz CG, Nutt JG, Stebbins GT (2008) The Unified Dyskinesia Rating Scale: presentation and clinimetric profile. Mov Disord 3:2398–2403
World Health Organization. Global report on falls prevention in older age. http://www.who.int/ageing/publications/Falls_prevention7March.pdfhttps://extranet.who.int/agefriendlyworld/wp-content/uploads/2014/06/WHo-Global-report-on-falls-prevention-in-older-age.pdf
Baruch MC, Kalantari K, Gerdt DW, Adkins CM (2014) Validation of the pulse decomposition analysis algorithm using central arterial blood pressure. Biomed Eng Online 13:96
Palma JA, Gomez-Esteban JC, Norcliffe-Kaufmann L, Martinez J, Tijero B, Berganzo K, Kaufmann H (2015) Orthostatic hypotension in Parkinson disease: how much you fall or how low you go? Mov Disord 30:639–645
Vallelonga F, Di Stefano C, Merola A, Romagnolo A, Sobrero G, Milazzo V, Burrello A, Burrello J, Zibetti M, Veglio F, Maule S (2019) Blood pressure circadian rhythm alterations in alpha-synucleinopathies. J Neurol 266:1141–1152
Mancini M, King L, Salarian A, Holmstrom L, McNames J, Horak FB (2011) Mobility lab to assess balance and gait with synchronized body-worn sensors. J Bioeng Biomed Sci Suppl 1:007
Heldman DA, Espay AJ, LeWitt PA, Giuffrida JP (2014) Clinician versus machine: reliability and responsiveness of motor endpoints in Parkinson's disease. Parkinsonism Relat Disord 20:590–595
Pulliam CL, Heldman DA, Brokaw EB, Mera TO, Mari ZK, Burack MA (2018) Continuous assessment of levodopa response in Parkinson’s disease using wearable motion sensors. IEEE Trans Biomed Eng 65(1):159–164
Heldman DA, Brokaw EB, Espay AJ, Revilla FJ, Riley DE, Mera TO, Giuffrida JP, Walter BL (2015) Wearable sensors for quantifying deep brain stimulation washout effects on gait in Parkinson’s disease. Mov Disord 30(1):S221
Yamamoto T, Smith CE, Suzuki Y, Kiyono K, Tanahashi T, Sakoda S, Morasso P, Nomura T (2015) Universal and individual characteristics of postural sway during quiet standing in healthy young adults. Physiol Rep 3:e12329
Mancini M, Salarian A, Carlson-Kuhta P, Zampieri C, King L, Chiari L, Horak FB (2012) ISway: a sensitive, valid and reliable measure of postural control. J Neuroeng Rehabil 9:59
Dewey DC, Miocinovic S, Bernstein I, Khemani P, Dewey RB 3rd, Querry R, Chitnis S, Dewey RB Jr (2014) Automated gait and balance parameters diagnose and correlate with severity in Parkinson disease. J Neurol Sci 345:131–138
Pal G, O'Keefe J, Robertson-Dick E, Bernard B, Anderson S, Hall D (2016) Global cognitive function and processing speed are associated with gait and balance dysfunction in Parkinson's disease. J Neuroeng Rehabil 13:94
El-Gohary M, Pearson S, McNames J, Mancini M, Horak F, Mellone S, Chiari L (2013) Continuous monitoring of turning in patients with movement disability. Sensors (Basel) 14:356–369
Washabaugh EP, Kalyanaraman T, Adamczyk PG, Claflin ES, Krishnan C (2017) Validity and repeatability of inertial measurement units for measuring gait parameters. Gait Posture 55:87–93
Grangeon M, Gauthier C, Duclos C, Lemay JF, Gagnon D (2015) Unsupported eyes closed sitting and quiet standing share postural control strategies in healthy individuals. Mot Control 19:10–24
Lord S, Galna B, Yarnall AJ, Coleman S, Burn D, Rochester L (2016) Predicting first fall in newly diagnosed Parkinson's disease: insights from a fall-naïve cohort. Mov Disord 31:1829–1836
Kao CC, Chiu HL, Liu D, Chan PT, Tseng IJ, Chen R, Niu SF, Chou KR (2018) Effect of interactive cognitive motor training on gait and balance among older adults: a randomized controlled trial. Int J Nurs Stud 82:121–128
Pilleri M, Facchini S, Gasparoli E, Biundo R, Bernardi L, Marchetti M, Formento P, Antonini A (2013) Cognitive and MRI correlates of orthostatic hypotension in Parkinson's disease. J Neurol 260:253–259
Hayashida K, Nishiooeda Y, Hirose Y, Ishida Y, Nishimura T (1996) Maladaptation of vascular response in frontal area of patients with orthostatic hypotension. J Nucl Med 37:1–4
Pilotto A, Romagnolo A, Tuazon JA, Vizcarra JA, Marsili L, Zibetti M, Rosso M, Rodriguez-Porcel F, Borroni B, Rizzetti MC, Rossi C, Vizcarra-Escobar D, Molano JR, Lopiano L, Ceravolo R, Masellis M, Espay AJ, Padovani A, Merola A (2019) Orthostatic hypotension and REM sleep behaviour disorder: impact on clinical outcomes in α-synucleinopathies. J Neurol Neurosurg Psychiatry 90:1257–1263
Durrieu G, Senard JM, Tran MA, Rascol A, Montastruc JL (1991) Effects of levodopa and bromocriptine on blood pressure and plasma catecholamines in parkinsonians. Clin Neuropharmacol 14:84–90
Haapaniemi TH, Kallio MA, Korpelainen JT, Suominen K, Tolonen U, Sotaniemi KA, Myllylä VV (2020) Levodopa, bromocriptine and selegiline modify cardiovascular responses in Parkinson's disease. J Neurol 247:868–874
Goldstein DS, Eldadah BA, Holmes C, Pechnik S, Moak J, Saleem A, Sharabi Y (2005) Neurocirculatory abnormalities in Parkinson disease with orthostatic hypotension: independence from levodopa treatment. Hypertension 46:1333–1339
Jost WH, Altmann C, Fiesel T, Becht B, Ringwald S, Hoppe T (2020) Influence of levodopa on orthostatic hypotension in Parkinson's disease. Neurol Neurochir Pol 54:200–203
Lim I, van Wegen E, Jones D, Rochester I, Nieuwboer A, Willems AM, Baker K, Hetherington V, Kwakkel G (2008) Identifying fallers with Parkinson's disease using home-based tests: who is at risk? Mov Disord 23:2411–2415
Espay AJ, Hausdorff JM, Sánchez-Ferro A, Klucken J, Merola A, Bonato P, Paul SS, Horak FB, Vizcarra JA, Mestre TA, Reilmann R, Nieuwboer A, Dorsey ER, Rochester L, Bloem BR, Maetzler W (2019) Movement Disorder Society Task Force on Technology. A roadmap for implementation of patient-centered digital outcome measures in Parkinson's disease obtained using mobile health technologies. Mov Disord 34:657–663
Acknowledgements
We acknowledge the contribution of patients who took part in this study and the healthcare professionals working at the Gardner’s Center for Movement Disorders at the University of Cincinnati.
Funding
This work has been supported by the National Institute of Health (NIH), Grant KL2 TR001426.
Author information
Authors and Affiliations
Contributions
AS contributed to concept and design of the manuscript, analyzed and interpreted data, and drafted/revised the manuscript for content. AKD analyzed and interpreted data and revised the manuscript for content. LM, AH, GS and DH contributed to acquisition of data, interpreted data, and revised the manuscript for content. SM, LL, CC, MV, and AJE interpreted data and revised the manuscript for content. AM helped in study concept and design, analyzed and interpreted data and drafted/revised the manuscript for content.
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Sturchio has no financial conflicts to disclose. Prof. Dwivedi is supported as a co-investigator by the NIH (1 R21 HL143030-01) and (R21 AI133207) grants. He is also currently serving as a statistician in CPRIT-funded studies (PP200006, PP190058, PP180003, and PP170068). Dr. Dwivedi is also an Adjunct Associate Professor in the department of neurology and rehabilitation medicine, University of Cincinnati. Dr. Marsili has no financial conflicts to disclose. Dr. Hadley owns stock in Great Lakes NeuroTechnologies and has received compensation for employment. Dr. Hadley has received grant funding from the NIH. Dr. Sobrero has no financial conflicts to disclose. Dr. Heldman serves on the board of directors of Great Lakes NeuroTechnologies. He also owns stock in Great Lakes NeuroTechnologies and has received compensation for employment. Dr. Maule has no financial conflicts to disclose. Dr. Lopiano has received grant support from Abbvie, Zambon and personal compensation from Abbvie, Zambon, DOC, Bial, UCB. Dr. Comi has received grant support from the “Agenzia Italiana del Farmaco” and from “Fondazione Cariplo”; and travel grants from Zambon S.P.A. and Mylan. Dr. Versino received academic fund support from Chiesi Farmaceutici SpA. Dr. Espay has received grant support from the NIH, Great Lakes Neurotechnologies and the Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Abbvie, TEVA, Impax, Acadia, Acorda, Cynapsus/Sunovion, Lundbeck, and USWorldMeds; publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer; and honoraria from Abbvie, UCB, USWorldMeds, Lundbeck, Acadia, the American Academy of Neurology, and the Movement Disorders Society. He serves as Associate Editor of the Journal of Clinical Movement Disorders and on the editorial boards of JAMA Neurology, the Journal of Parkinson’s Disease and Parkinsonism and Related Disorders. Dr. Merola is supported by NIH (KL2 TR001426) and has received speaker honoraria from CSL Behring, Abbvie, Abbott, Theravance, and Cynapsus Therapeutics. He has received grant support from Lundbeck and Abbvie.
Ethics approval
The study received IRB/ethics committee approval at all participating centers and was conducted in accordance with the Good Clinical Practice and the International Conference on Harmonization guidelines and any applicable national and local regulations. The authors declare that they acted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Data access and responsibility statement
A. Merola had full access to all the data in the study and takes responsibility for the integrity of the data, the accuracy of the data analysis, and the conduct of the research. He has the right to publish any and all data, separate and apart from the guidance of any sponsor.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sturchio, A., Dwivedi, A.K., Marsili, L. et al. Kinematic but not clinical measures predict falls in Parkinson-related orthostatic hypotension. J Neurol 268, 1006–1015 (2021). https://doi.org/10.1007/s00415-020-10240-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-10240-8