Abstract
Background
Recent studies have suggested the presence of a significant atrophy affecting the cerebellar cortex in Friedreich ataxia (FRDA) patients, an area of the brain long considered to be relatively spared by neurodegenerative phenomena. Cognitive deficits, which occur in FRDA patients, have been associated with cerebellar volume loss in other conditions. The aim of this study was to investigate the correlation between cerebellar volume and cognition in FRDA.
Methods
Nineteen FRDA patients and 20 healthy controls (HC) were included in this study and evaluated via a neuropsychological examination. Cerebellar global and lobular volumes were computed using the Spatially Unbiased Infratentorial Toolbox (SUIT). Furthermore, a cerebellar voxel-based morphometry (VBM) analysis was also carried out. Correlations between MRI metrics and clinical data were tested via partial correlation analysis.
Results
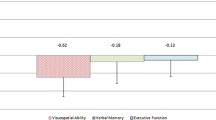
FRDA patients showed a significant reduction of the total cerebellar volume (p = 0.004), significantly affecting the Lobule IX (p = 0.001). At the VBM analysis, we found a cluster of significant reduced GM density encompassing the entire lobule IX (p = 0.003). When correlations were probed, we found a direct correlation between Lobule IX volume and impaired visuo-spatial functions (r = 0.58, p = 0.02), with a similar correlation that was found between the same altered function and results obtained at the VBM (r = 0.52; p = 0.03).
Conclusions
With two different image analysis techniques, we confirmed the presence of cerebellar volume loss in FRDA, mainly affecting the posterior lobe. In particular, Lobule IX atrophy correlated with worse visuo-spatial abilities, further expanding our knowledge about the physiopathology of cognitive impairment in FRDA.



Similar content being viewed by others
References
Campuzano V, Montermini L, Molto MD, Pianese L, Cossee M, Cavalcanti F, Monros E, Rodius F, Duclos F, Monticelli A, Zara F, Canizares J, Koutnikova H, Bidichandani SI, Gellera C, Brice A, Trouillas P, De Michele G, Filla A, De Frutos R, Palau F, Patel PI, Di Donato S, Mandel JL, Cocozza S, Koenig M, Pandolfo M (1996) Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271(5254):1423–1427
Koeppen AH, Mazurkiewicz JE (2013) Friedreich ataxia: neuropathology revised. J Neuropathol Exp Neurol 72(2):78–90. https://doi.org/10.1097/NEN.0b013e31827e5762
Solbach K, Kraff O, Minnerop M, Beck A, Schols L, Gizewski ER, Ladd ME, Timmann D (2014) Cerebellar pathology in Friedreich’s ataxia: atrophied dentate nuclei with normal iron content. Neuroimage Clin 6:93–99. https://doi.org/10.1016/j.nicl.2014.08.018
Ward PGD, Harding IH, Close TG, Corben LA, Delatycki MB, Storey E, Georgiou-Karistianis N, Egan GF (2019) Longitudinal evaluation of iron concentration and atrophy in the dentate nuclei in Friedreich ataxia. Mov Disord 34(3):335–343. https://doi.org/10.1002/mds.27606
Mascalchi M (2013) The cerebellum looks normal in Friedreich ataxia. AJNR Am J Neuroradiol 34(2):E22. https://doi.org/10.3174/ajnr.A3480
Ormerod IE, Harding AE, Miller DH, Johnson G, MacManus D, du Boulay EP, Kendall BE, Moseley IF, McDonald WI (1994) Magnetic resonance imaging in degenerative ataxic disorders. J Neurol Neurosurg Psychiatry 57(1):51–57. https://doi.org/10.1136/jnnp.57.1.51
Della Nave R, Ginestroni A, Giannelli M, Tessa C, Salvatore E, Salvi F, Dotti MT, De Michele G, Piacentini S, Mascalchi M (2008) Brain structural damage in Friedreich’s ataxia. J Neurol Neurosurg Psychiatry 79(1):82–85. https://doi.org/10.1136/jnnp.2007.124297
Selvadurai LP, Harding IH, Corben LA, Stagnitti MR, Storey E, Egan GF, Delatycki MB, Georgiou-Karistianis N (2016) Cerebral and cerebellar grey matter atrophy in Friedreich ataxia: the IMAGE-FRDA study. J Neurol 263(11):2215–2223. https://doi.org/10.1007/s00415-016-8252-7
Vavla M, Arrigoni F, Nordio A, De Luca A, Pizzighello S, Petacchi E, Paparella G, D’Angelo MG, Brighina E, Russo E, Fantin M, Colombo P, Martinuzzi A (2018) Functional and structural brain damage in Friedreich’s ataxia. Front Neurol 9:747. https://doi.org/10.3389/fneur.2018.00747
Pandolfo M (2009) Friedreich ataxia: the clinical picture. J Neurol 256(Suppl 1):3–8. https://doi.org/10.1007/s00415-009-1002-3
Cocozza S, Costabile T, Tedeschi E, Abate F, Russo C, Liguori A, Del Vecchio W, Paciello F, Quarantelli M, Filla A, Brunetti A, Sacca F (2018) Cognitive and functional connectivity alterations in Friedreich’s ataxia. Ann Clin Transl Neurol 5(6):677–686. https://doi.org/10.1002/acn3.555
Noroozian M (2014) The role of the cerebellum in cognition: beyond coordination in the central nervous system. Neurol Clin 32(4):1081–1104. https://doi.org/10.1016/j.ncl.2014.07.005
Koziol LF, Budding D, Andreasen N, D’Arrigo S, Bulgheroni S, Imamizu H, Ito M, Manto M, Marvel C, Parker K, Pezzulo G, Ramnani N, Riva D, Schmahmann J, Vandervert L, Yamazaki T (2014) Consensus paper: the cerebellum’s role in movement and cognition. Cerebellum 13(1):151–177. https://doi.org/10.1007/s12311-013-0511-x
Stoodley CJ, Schmahmann JD (2010) Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46(7):831–844. https://doi.org/10.1016/j.cortex.2009.11.008
Deverett B, Koay SA, Oostland M, Wang SS (2018) Cerebellar involvement in an evidence-accumulation decision-making task. Elife. https://doi.org/10.7554/eLife.36781
Chen Y, Kumfor F, Landin-Romero R, Irish M, Hodges JR, Piguet O (2018) Cerebellar atrophy and its contribution to cognition in frontotemporal dementias. Ann Neurol 84(1):98–109. https://doi.org/10.1002/ana.25271
Yang H, Wang N, Luo X, Lv H, Liu H, Li Y, Fan G (2019) Cerebellar atrophy and its contribution to motor and cognitive performance in multiple system atrophy. Neuroimage Clin 23:101891. https://doi.org/10.1016/j.nicl.2019.101891
Cocozza S, Petracca M, Mormina E, Buyukturkoglu K, Podranski K, Heinig MM, Pontillo G, Russo C, Tedeschi E, Russo CV, Costabile T, Lanzillo R, Harel A, Klineova S, Miller A, Brunetti A, Morra VB, Lublin F, Inglese M (2017) Cerebellar lobule atrophy and disability in progressive MS. J Neurol Neurosurg Psychiatry 88(12):1065–1072. https://doi.org/10.1136/jnnp-2017-316448
Kansal K, Yang Z, Fishman AM, Sair HI, Ying SH, Jedynak BM, Prince JL, Onyike CU (2017) Structural cerebellar correlates of cognitive and motor dysfunctions in cerebellar degeneration. Brain 140(3):707–720. https://doi.org/10.1093/brain/aww327
Olivito G, Lupo M, Iacobacci C, Clausi S, Romano S, Masciullo M, Molinari M, Cercignani M, Bozzali M, Leggio M (2018) Structural cerebellar correlates of cognitive functions in spinocerebellar ataxia type 2. J Neurol 265(3):597–606. https://doi.org/10.1007/s00415-018-8738-6
Criscuolo C, Mancini P, Menchise V, Sacca F, De Michele G, Banfi S, Filla A (2005) Very late onset in ataxia oculomotor apraxia type I. Ann Neurol 57(5):777. https://doi.org/10.1002/ana.20463
Pane C, Costabile T, Salvati A, Aurisicchio DL, Abate F, Liguori A, Paciello F, Peluso S, Manganelli F, De Michele G, Filla A, Sacca F (2018) Adult normative values for the PATA rate test. J Neurol 265(5):1102–1105. https://doi.org/10.1007/s00415-018-8820-0
Schmitz-Hubsch T, Coudert M, Bauer P, Giunti P, Globas C, Baliko L, Filla A, Mariotti C, Rakowicz M, Charles P, Ribai P, Szymanski S, Infante J, van de Warrenburg BP, Durr A, Timmann D, Boesch S, Fancellu R, Rola R, Depondt C, Schols L, Zdienicka E, Kang JS, Dohlinger S, Kremer B, Stephenson DA, Melegh B, Pandolfo M, di Donato S, du Montcel ST, Klockgether T (2008) Spinocerebellar ataxia types 1, 2, 3, and 6: disease severity and nonataxia symptoms. Neurology 71(13):982–989. https://doi.org/10.1212/01.wnl.0000325057.33666.72
Sacca F, Costabile T, Abate F, Liguori A, Paciello F, Pane C, De Rosa A, Manganelli F, De Michele G, Filla A (2018) Normalization of timed neuropsychological tests with the PATA rate and nine-hole pegboard tests. J Neuropsychol 12(3):471–483. https://doi.org/10.1111/jnp.12125
D’Mello AM, Crocetti D, Mostofsky SH, Stoodley CJ (2015) Cerebellar gray matter and lobular volumes correlate with core autism symptoms. Neuroimage Clin 7:631–639. https://doi.org/10.1016/j.nicl.2015.02.007
Clemm von Hohenberg C, Schocke MF, Wigand MC, Nachbauer W, Guttmann CR, Kubicki M, Shenton ME, Boesch S, Egger K (2013) Radial diffusivity in the cerebellar peduncles correlates with clinical severity in Friedreich ataxia. Neurol Sci 34(8):1459–1462. https://doi.org/10.1007/s10072-013-1402-0
Della Nave R, Ginestroni A, Diciotti S, Salvatore E, Soricelli A, Mascalchi M (2011) Axial diffusivity is increased in the degenerating superior cerebellar peduncles of Friedreich’s ataxia. Neuroradiology 53(5):367–372. https://doi.org/10.1007/s00234-010-0807-1
Della Nave R, Ginestroni A, Tessa C, Salvatore E, Bartolomei I, Salvi F, Dotti MT, De Michele G, Piacentini S, Mascalchi M (2008) Brain white matter tracts degeneration in Friedreich ataxia. An in vivo MRI study using tract-based spatial statistics and voxel-based morphometry. Neuroimage 40(1):19–25. https://doi.org/10.1016/j.neuroimage.2007.11.050
Harding IH, Corben LA, Storey E, Egan GF, Stagnitti MR, Poudel GR, Delatycki MB, Georgiou-Karistianis N (2016) Fronto-cerebellar dysfunction and dysconnectivity underlying cognition in Friedreich ataxia: the IMAGE-FRDA study. Hum Brain Mapp 37(1):338–350. https://doi.org/10.1002/hbm.23034
Harding IH, Raniga P, Delatycki MB, Stagnitti MR, Corben LA, Storey E, Georgiou-Karistianis N, Egan GF (2016) Tissue atrophy and elevated iron concentration in the extrapyramidal motor system in Friedreich ataxia: the IMAGE-FRDA study. J Neurol Neurosurg Psychiatry 87(11):1261–1263. https://doi.org/10.1136/jnnp-2015-312665
Rezende TJ, Silva CB, Yassuda CL, Campos BM, D’Abreu A, Cendes F, Lopes-Cendes I, Franca MC Jr (2016) Longitudinal magnetic resonance imaging study shows progressive pyramidal and callosal damage in Friedreich’s ataxia. Mov Disord 31(1):70–78. https://doi.org/10.1002/mds.26436
Selvadurai LP, Harding IH, Corben LA, Georgiou-Karistianis N (2018) Cerebral abnormalities in Friedreich ataxia: a review. Neurosci Biobehav Rev 84:394–406. https://doi.org/10.1016/j.neubiorev.2017.08.006
Stefanescu MR, Dohnalek M, Maderwald S, Thurling M, Minnerop M, Beck A, Schlamann M, Diedrichsen J, Ladd ME, Timmann D (2015) Structural and functional MRI abnormalities of cerebellar cortex and nuclei in SCA3, SCA6 and Friedreich’s ataxia. Brain 138(Pt 5):1182–1197. https://doi.org/10.1093/brain/awv064
Deluca C, Golzar A, Santandrea E, Lo Gerfo E, Estocinova J, Moretto G, Fiaschi A, Panzeri M, Mariotti C, Tinazzi M, Chelazzi L (2014) The cerebellum and visual perceptual learning: evidence from a motion extrapolation task. Cortex 58:52–71. https://doi.org/10.1016/j.cortex.2014.04.017
Handel B, Thier P, Haarmeier T (2009) Visual motion perception deficits due to cerebellar lesions are paralleled by specific changes in cerebro-cortical activity. J Neurosci 29(48):15126–15133. https://doi.org/10.1523/JNEUROSCI.3972-09.2009
Jokisch D, Troje NF, Koch B, Schwarz M, Daum I (2005) Differential involvement of the cerebellum in biological and coherent motion perception. Eur J Neurosci 21(12):3439–3446. https://doi.org/10.1111/j.1460-9568.2005.04145.x
Salmi J, Pallesen KJ, Neuvonen T, Brattico E, Korvenoja A, Salonen O, Carlson S (2010) Cognitive and motor loops of the human cerebro-cerebellar system. J Cogn Neurosci 22(11):2663–2676. https://doi.org/10.1162/jocn.2009.21382
Stephen R, Elizabeth Y, Christophe H (2018) Participation of the caudal cerebellar lobule IX to the dorsal attentional network. Cerebellum Ataxias 5:9. https://doi.org/10.1186/s40673-018-0088-8
Lupo M, Olivito G, Siciliano L, Masciullo M, Bozzali M, Molinari M, Leggio M (2018) Development of a psychiatric disorder linked to cerebellar lesions. Cerebellum 17(4):438–446. https://doi.org/10.1007/s12311-018-0926-5
Steinlin M (2008) Cerebellar disorders in childhood: cognitive problems. Cerebellum 7(4):607–610. https://doi.org/10.1007/s12311-008-0083-3
Jacobi H, Reetz K, du Montcel ST, Bauer P, Mariotti C, Nanetti L, Rakowicz M, Sulek A, Durr A, Charles P, Filla A, Antenora A, Schols L, Schicks J, Infante J, Kang JS, Timmann D, Di Fabio R, Masciullo M, Baliko L, Melegh B, Boesch S, Burk K, Peltz A, Schulz JB, Dufaure-Gare I, Klockgether T (2013) Biological and clinical characteristics of individuals at risk for spinocerebellar ataxia types 1, 2, 3, and 6 in the longitudinal RISCA study: analysis of baseline data. Lancet Neurol 12(7):650–658. https://doi.org/10.1016/S1474-4422(13)70104-2
Cocozza S, Sacca F, Cervo A, Marsili A, Russo CV, Giorgio SM, De Michele G, Filla A, Brunetti A, Quarantelli M (2015) Modifications of resting state networks in spinocerebellar ataxia type 2. Mov Disord 30(10):1382–1390. https://doi.org/10.1002/mds.26284
Jung BC, Choi SI, Du AX, Cuzzocreo JL, Ying HS, Landman BA, Perlman SL, Baloh RW, Zee DS, Toga AW, Prince JL, Ying SH (2012) MRI shows a region-specific pattern of atrophy in spinocerebellar ataxia type 2. Cerebellum 11(1):272–279. https://doi.org/10.1007/s12311-011-0308-8
Hernandez-Castillo CR, Diaz R, Campos-Romo A, Fernandez-Ruiz J (2017) Neural correlates of ataxia severity in spinocerebellar ataxia type 3/Machado-Joseph disease. Cerebellum Ataxias 4:7. https://doi.org/10.1186/s40673-017-0065-7
Rub U, Brunt ER, Seidel K, Gierga K, Mooy CM, Kettner M, Van Broeckhoven C, Bechmann I, La Spada AR, Schols L, den Dunnen W, de Vos RA, Deller T (2008) Spinocerebellar ataxia type 7 (SCA7): widespread brain damage in an adult-onset patient with progressive visual impairments in comparison with an adult-onset patient without visual impairments. Neuropathol Appl Neurobiol 34(2):155–168. https://doi.org/10.1111/j.1365-2990.2007.00882.x
Hernandez-Castillo CR, King M, Diedrichsen J, Fernandez-Ruiz J (2018) Unique degeneration signatures in the cerebellar cortex for spinocerebellar ataxias 2, 3, and 7. Neuroimage Clin 20:931–938. https://doi.org/10.1016/j.nicl.2018.09.026
Reetz K, Dogan I, Rolfs A, Binkofski F, Schulz JB, Laird AR, Fox PT, Eickhoff SB (2012) Investigating function and connectivity of morphometric findings-exemplified on cerebellar atrophy in spinocerebellar ataxia 17 (SCA17). Neuroimage 62(3):1354–1366. https://doi.org/10.1016/j.neuroimage.2012.05.058
Hoche F, Frankenberg E, Rambow J, Theis M, Harding JA, Qirshi M, Seidel K, Barbosa-Sicard E, Porto L, Schmahmann JD, Kieslich M (2014) Cognitive phenotype in ataxia-telangiectasia. Pediatr Neurol 51(3):297–310. https://doi.org/10.1016/j.pediatrneurol.2014.04.027
Gold JI, Shadlen MN (2007) The neural basis of decision making. Annu Rev Neurosci 30:535–574. https://doi.org/10.1146/annurev.neuro.29.051605.113038
Kelly RM, Strick PL (2003) Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci 23(23):8432–8444
Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD (2009) Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci 29(26):8586–8594. https://doi.org/10.1523/JNEUROSCI.1868-09.2009
Sang L, Qin W, Liu Y, Han W, Zhang Y, Jiang T, Yu C (2012) Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. Neuroimage 61(4):1213–1225. https://doi.org/10.1016/j.neuroimage.2012.04.011
Liu J, Wang Q, Liu F, Song H, Liang X, Lin Z, Hong W, Yang S, Huang J, Zheng G, Tao J, Chen LD (2017) Altered functional connectivity in patients with post-stroke memory impairment: a resting fMRI study. Exp Ther Med 14(3):1919–1928. https://doi.org/10.3892/etm.2017.4751
Jerde TA, Merriam EP, Riggall AC, Hedges JH, Curtis CE (2012) Prioritized maps of space in human frontoparietal cortex. J Neurosci 32(48):17382–17390. https://doi.org/10.1523/JNEUROSCI.3810-12.2012
Guell X, Schmahmann JD, Gabrieli J, Ghosh SS (2018) Functional gradients of the cerebellum. Elife. https://doi.org/10.7554/eLife.36652
Lindig T, Bender B, Kumar VJ, Hauser TK, Grodd W, Brendel B, Just J, Synofzik M, Klose U, Scheffler K, Ernemann U, Schols L (2019) Pattern of cerebellar atrophy in Friedreich’s ataxia-using the SUIT template. Cerebellum 18(3):435–447. https://doi.org/10.1007/s12311-019-1008-z
Nachbauer W, Bodner T, Boesch S, Karner E, Eigentler A, Neier L, Benke T, Delazer M (2014) Friedreich ataxia: executive control is related to disease onset and GAA repeat length. Cerebellum 13(1):9–16. https://doi.org/10.1007/s12311-013-0513-8
Nieto A, Correia R, de Nobrega E, Monton F, Hess S, Barroso J (2012) Cognition in Friedreich ataxia. Cerebellum 11(4):834–844. https://doi.org/10.1007/s12311-012-0363-9
Huh YE, Kim JS (2011) Patterns of spontaneous and head-shaking nystagmus in cerebellar infarction: imaging correlations. Brain 134(Pt 12):3662–3671. https://doi.org/10.1093/brain/awr269
Lee SH, Park SH, Kim JS, Kim HJ, Yunusov F, Zee DS (2014) Isolated unilateral infarction of the cerebellar tonsil: ocular motor findings. Ann Neurol 75(3):429–434
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15(1):273–289. https://doi.org/10.1006/nimg.2001.0978
Funding
SC: Fees for speaking from Sanofi-Genzyme. TC: None. GP: Fees for speaking from Sanofi-Genzyme. ML: None. CR: Fees for speaking from Sanofi-Genzyme. LR: None. CP: None. AF: None. AB: None. FS: Fees for speaking from Biogen, Mylan, Novartis, Roche, Sanofi, Teva; Fees for Advisory boards from: Almirall, Argenx, Avexis, Forward Pharma, Merk, Novartis, Pomona, Roche, Sanofi. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
SC: Conception, organization and execution of the research, writing of the manuscript. TC: Organization and execution of the research, writing and review of the manuscript. GP: Organization and execution of the research, writing of the manuscript. ML: Conception and execution of the research, review of the manuscript. CR: Conception of the research, writing and review of the manuscript. LR: Organization and execution of the research, review of the manuscript. CP: Organization and execution of the research, review of the manuscript. AF: Conception of the research, review and critique of the manuscript. AB: Writing, review and critique of the manuscript. FS: Design, execution, writing, review and critique of the manuscript
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cocozza, S., Costabile, T., Pontillo, G. et al. Cerebellum and cognition in Friedreich ataxia: a voxel-based morphometry and volumetric MRI study. J Neurol 267, 350–358 (2020). https://doi.org/10.1007/s00415-019-09582-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09582-9