Abstract
Background
The association of Lewy bodies (LBs) with olfactory dysfunction was investigated in community-dwelling elders without clinical Parkinson’s disease (PD) using the 12-item Brief Smell Identification Test (BSIT), a standard measure of odor identification.
Methods
280 participants in the Rush Memory and Aging Project completed the BSIT annually. Lewy bodies were detected in 13 brain regions by immunohistochemistry and were assigned to the Braak PD stages 1–6.
Results
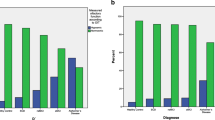
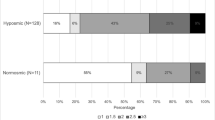
Of the 280 participants, 101 (36.1%) had LBs which were maximal in the olfactory bulb and tract (85.1%) and least in Heschl’s cortex (21.8%). Due to the small number of cases in Braak PD stages 2, 3 and 5, the distribution of LBs in the 6 Braak PD stages was contracted into 3 main LB stages: (1) LBs in olfactory bulbs and dorsal motor nucleus of vagus, (2) further extension of LBs to limbic and other brainstem regions and (3) additional extension of LBs to neocortical areas. MMSE, global cognition and odor test scores were lower and frequency of dementia was higher at the time of the last valid BSIT, in cases with LBs as compared to those without LBs. Linear regression analyses showed that LBs were associated with impaired olfaction. However, on stratification of LBs into 3 stages, only the stage 3 cases were independently associated with impaired olfaction.
Conclusion
Although LB pathology was detected in olfactory bulbs in the early stage of LB progression (stage 1), the strongest association of LBs with olfactory dysfunction was observed in the late pathological stage (stage 3) when LBs extended to neocortical areas.

Similar content being viewed by others
References
Boyce JM, Shone GR (2006) Effects of ageing on smell and taste. Postgrad Med J 82:239–241
Landis BN, Konnerth CG, Hummel T (2004) A study on the frequency of olfactory dysfunction. Laryngoscope 114:1764–1769
Rouby C, Thomas-Danguin T, Vigouroux M, Ciuperca G, Jiang T, Alexanian J, Barges M, Gallice I, Degraix JL, Sicard G (2011) The lyon clinical olfactory test: validation and measurement of hyposmia and anosmia in healthy and diseased populations. Int J Otolaryngol 2011:203805
Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM (2002) Prevalence of olfactory impairment in older adults. JAMA 288:2307–2312
Attems J, Walker L, Jellinger KA (2014) Olfactory bulb involvement in neurodegenerative diseases. Acta Neuropathol 127:459–475
Doty RL (2012) Olfactory dysfunction in Parkinson disease. Nat Rev Neurol 8:329–339
Driver-Dunckley E, Adler CH, Hentz JG, Dugger BN, Shill HA, Caviness JN, Sabbagh MN, Beach TG (2014) Olfactory dysfunction in incidental Lewy body disease and Parkinson's disease. Parkinsonism Relat Disord 20:1260–1262
Hawkes CH, Shephard BC, Daniel SE (1997) Olfactory dysfunction in Parkinson's disease. J Neurol Neurosurg Psychiatry 62:436–446
Mesholam RI, Moberg PJ, Mahr RN, Doty RL (1998) Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer's and Parkinson's diseases. Arch Neurol 55:84–90
Doty RL, Reyes PF, Gregor T (1987) Presence of both odor identification and detection deficits in Alzheimer's disease. Brain Res Bull 18:597–600
Koss E, Weiffenbach JM, Haxby JV, Friedland RP (1988) Olfactory detection and identification performance are dissociated in early Alzheimer's disease. Neurology 38:1228–1232
Kovacs T, Cairns NJ, Lantos PL (2001) Olfactory centres in Alzheimer's disease: olfactory bulb is involved in early Braak's stages. NeuroReport 12:285–288
Wilson RS, Arnold SE, Schneider JA, Tang Y, Bennett DA (2007) The relationship between cerebral Alzheimer's disease pathology and odour identification in old age. J Neurol Neurosurg Psychiatry 78:30–35
Doty RL, Deems DA, Stellar S (1988) Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology 38:1237–1244
Hawkes C (2006) Olfaction in neurodegenerative disorder. Adv Otorhinolaryngol 63:133–151
Braak H, Del TK, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 24:197–211
Jellinger KA (2003) Alpha-synuclein pathology in Parkinson's and Alzheimer's disease brain: incidence and topographic distribution–a pilot study. Acta Neuropathol 106:191–201
Kranick SM, Duda JE (2008) Olfactory dysfunction in Parkinson's disease. Neurosignals 16:35–40
Tsuboi Y, Wszolek ZK, Graff-Radford NR, Cookson N, Dickson DW (2003) Tau pathology in the olfactory bulb correlates with Braak stage, Lewy body pathology and apolipoprotein epsilon4. Neuropathol Appl Neurobiol 29:503–510
Beach TG, White CL III, Hladik CL, Sabbagh MN, Connor DJ, Shill HA, Sue LI, Sasse J, Bachalakuri J, Henry-Watson J, Akiyama H, Adler CH (2009) Olfactory bulb alpha-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta Neuropathol 117:169–174
Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, Eschbacher J, White CL III, Akiyama H, Caviness J, Shill HA, Connor DJ, Sabbagh MN, Walker DG (2009) Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 117:613–634
Bloch A, Probst A, Bissig H, Adams H, Tolnay M (2006) Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol 32:284–295
Buchman AS, Nag S, Leurgans SE, Miller J, VanderHorst VGJM, Bennett DA, Schneider JA (2018) Spinal Lewy body pathology in older adults without an antemortem diagnosis of Parkinson's disease. Brain Pathol 28:560–568
Sengoku R, Saito Y, Ikemura M, Hatsuta H, Sakiyama Y, Kanemaru K, Arai T, Sawabe M, Tanaka N, Mochizuki H, Inoue K, Murayama S (2008) Incidence and extent of Lewy body-related alpha-synucleinopathy in aging human olfactory bulb. J Neuropathol Exp Neurol 67:1072–1083
Dickson DW, Fujishiro H, DelleDonne A, Menke J, Ahmed Z, Klos KJ, Josephs KA, Frigerio R, Burnett M, Parisi JE, Ahlskog JE (2008) Evidence that incidental Lewy body disease is pre-symptomatic Parkinson's disease. Acta Neuropathol 115:437–444
Jellinger KA (2012) Neuropathology of sporadic Parkinson's disease: evaluation and changes of concepts. Mov Disord 27:8–30
Silveira-Moriyama L, Holton J, Kingsbury A, Ayling H, Petrie A, Sterlacci W, Poewe W, Maier H, Lees AJ, Revesz T (2007) The primary olfactory cortex in idiopathic Parkinson's disease (IPD) and incidental Lewy body disease (ILBD). Mov Disord 22:S91
Hubbard PS, Esiri MM, Reading M, McShane R, Nagy Z (2007) Alpha-synuclein pathology in the olfactory pathways of dementia patients. J Anat 211:117–124
McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del ST, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology 65:1863–1872
Outeiro TF, Koss DJ, Erskine D, Walker L, Kurzawa-Akanbi M, Burn D, Donaghy P, Morris C, Taylor JP, Thomas A, Attems J, McKeith I (2019) Dementia with Lewy bodies: an update and outlook. Mol Neurodegener 14:5. https://doi.org/10.1186/s13024-019-0306-8
Doty RL, Shaman P, Dann M (1984) Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav 32:489–502
Wilson RS, Yu L, Schneider JA, Arnold SE, Buchman AS, Bennett DA (2011) Lewy bodies and olfactory dysfunction in old age. Chem Senses 36:367–373
Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA (2018) Religious orders study and rush memory and aging project. J Alzheimers Dis 64:S161–S189
Doty RL, Frye RE, Agrawal U (1989) Internal consistency reliability of the fractionated and whole University of Pennsylvania Smell Identification Test. Percept Psychophys 45:381–384
Doty RL, Marcus A, Lee WW (1996) Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT). Laryngoscope 106:353–356
Nag S, Yu L, Capuano AW, Wilson RS, Leurgans SE, Bennett DA, Schneider JA (2015) Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol 77:942–952
Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA (2006) Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology 67:441–445
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34:939–944
Bennett DA, Shannon KM, Beckett LA, Goetz CG, Wilson RS (1997) Metric properties of nurses' ratings of parkinsonian signs with a modified unified parkinson's disease rating scale. Neurology 49:1580–1587
Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA (2004) Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology 62:1148–1155
Buchman AS, Shulman JM, Nag S, Leurgans SE, Arnold SE, Morris MC, Schneider JA, Bennett DA (2012) Nigral pathology and parkinsonian signs in elders without Parkinson disease. Ann Neurol 71:258–266
Schneider JA, Arvanitakis Z, Yu L, Boyle PA, Leurgans SE, Bennett DA (2012) Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain 135:3005–3014
Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA (2010) Neurodegenerative basis of age-related cognitive decline. Neurology 75:1070–1078
Wilson RS, Arnold SE, Tang Y, Bennett DA (2006) Odor identification and decline in different cognitive domains in old age. Neuroepidemiology 26:61–67
Jellinger KA (2004) Lewy body-related alpha-synucleinopathy in the aged human brain. J Neural Transm (Vienna ) 111:1219–1235
Attems J, Jellinger KA (2008) The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson's disease. Neuropathol Appl Neurobiol 34:466–467
Jellinger KA (2008) A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol 116:1–16
Parkkinen L, Pirttila T, Alafuzoff I (2008) Applicability of current staging/categorization of alpha-synuclein pathology and their clinical relevance. Acta Neuropathol 115:399–407
Zaccai J, Brayne C, McKeith I, Matthews F, Ince PG (2008) Patterns and stages of alpha-synucleinopathy: Relevance in a population-based cohort. Neurology 70:1042–1048
Rey NL, Steiner JA, Maroof N, Luk KC, Madaj Z, Trojanowski JQ, Lee VM, Brundin P (2016) Widespread transneuronal propagation of alpha-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson's disease. J Exp Med 213:1759–1778
Braak H, Del TK (2017) Neuropathological staging of brain pathology in Sporadic Parkinson's disease: separating the wheat from the Chaff. J Parkinsons Dis 7:S71–S85
Rey NL, Wesson DW, Brundin P (2018) The olfactory bulb as the entry site for prion-like propagation in neurodegenerative diseases. Neurobiol Dis 109:226–248
Cersosimo MG (2018) Propagation of alpha-synuclein pathology from the olfactory bulb: possible role in the pathogenesis of dementia with Lewy bodies. Cell Tissue Res 373:233–243
Doty RL (2012) Olfaction in Parkinson's disease and related disorders. Neurobiol Dis 46:527–552
Patel RM, Pinto JM (2014) Olfaction: anatomy, physiology, and disease. Clin Anat 27:54–60
Hedner M, Larsson M, Arnold N, Zucco GM, Hummel T (2010) Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J Clin Exp Neuropsychol 32:1062–1067
Yang J, Pinto JM (2016) The epidemiology of olfactory disorders. Curr Otorhinolaryngol Rep 4:130–141
Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK (2015) The rate of age-related olfactory decline among the general population of older U.S. adults. J Gerontol A Biol Sci Med Sci 70:1435–1441
Atighechi S, Salari H, Baradarantar MH, Jafari R, Karimi G, Mirjali M (2009) A comparative study of brain perfusion single-photon emission computed tomography and magnetic resonance imaging in patients with post-traumatic anosmia. Am J Rhinol Allergy 23:409–412
Collet S, Grulois V, Bertrand B, Rombaux P (2009) Post-traumatic olfactory dysfunction: a cohort study and update. B-ENT 5(Suppl 13):97–107
Pieri L, Madiona K, Melki R (2016) Structural and functional properties of prefibrillar alpha-synuclein oligomers. Sci Rep 6:24526
Bengoa-Vergniory N, Roberts RF, Wade-Martins R, Alegre-Abarrategui J (2017) Alpha-synuclein oligomers: a new hope. Acta Neuropathol 134:819–838
Pieri L, Madiona K, Bousset L, Melki R (2012) Fibrillar alpha-synuclein and huntingtin exon 1 assemblies are toxic to the cells. Biophys J 102:2894–2905
Luk KC, Song C, O'Brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VM (2009) Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci U S A 106:20051–20056
Steiner JA, Quansah E, Brundin P (2018) The concept of alpha-synuclein as a prion-like protein: ten years after. Cell Tissue Res 373:161–173
Chartier S, Duyckaerts C (2018) Is Lewy pathology in the human nervous system chiefly an indicator of neuronal protection or of toxicity? Cell Tissue Res 373:149–160
Luk KC, Kehm VM, Zhang B, O'Brien P, Trojanowski JQ, Lee VM (2012) Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med 209:975–986
Attems J, Lintner F, Jellinger KA (2005) Olfactory involvement in aging and Alzheimer's disease: an autopsy study. J Alzheimers Dis 7:149–157
Acknowledgements
We thank all the participants of the Rush Memory and Aging Project and the staff of Rush Alzheimer’s Disease Center including Er-Yun Chen and Alysha Hodges.
Funding
This work was supported by National Institutes of Health, National Institute on Aging (R01AG017917, R01NS078009, R01AG047976) and the Illinois Department of Public Health. The funding organizations had no role in the design or conduct of the study, collection, management, analysis or interpretation of the data; or preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
SN and RSW were involved in conception and design, analysis and interpretation of data. LY made substantial contributions to the design of the statistical analysis of the data. SN and VGV oversaw the acquisition of data and SN drafted the manuscript and revised it following review by all the authors. All the authors gave final approval of the version to be published. SN and RSW are accountable for all aspects of the work and will ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing financial interests.
Ethics approval and consent to participate
Autopsied participants were from a longitudinal clinical–pathological study of aging and dementia, the Rush Memory and Aging Project. A signed, informed consent was obtained from each participant for an annual clinical evaluation and for brain donation. This study was approved by the Institutional Review Board of Rush University Medical Center and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Rights and permissions
About this article
Cite this article
Nag, S., Yu, L., VanderHorst, V.G. et al. Neocortical Lewy bodies are associated with impaired odor identification in community-dwelling elders without clinical PD. J Neurol 266, 3108–3118 (2019). https://doi.org/10.1007/s00415-019-09540-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09540-5