Abstract
Background
The increase in disease-modifying drugs (DMDs) allows individualization of treatment in relapsing multiple sclerosis (RMS); however, the long-term impact of different treatment sequences is not well established. This is particularly relevant for MS patients who may need to postpone more aggressive DMD strategies.
Objective
To evaluate different therapeutic strategies and their long-term outcomes, measured as relapses and confirmed disability progression (CDP), in MS ‘real-world’ settings.
Methods
Multicentre, observational, retrospectively acquired cohort study evaluating the long-term impact of different treatment strategies on disability outcomes in patients with RMS in the Italian MS Register.
Results
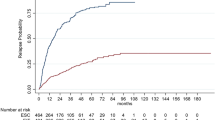
We evaluated 1152 RMS-naïve patients after propensity-score adjustment. Patients included were receiving: interferon beta-1a (IFN-β1a) 44 µg switching to fingolimod (FTY; IFN-switchers; n = 97); FTY only (FTY-stayers; n = 157); IFN-β1a only (IFN-stayers; n = 849). CDP and relapses did not differ between FTY-stayers and IFN-switchers [HR (95% CI) 0.99 (0.48–2.04), p = 0.98 and 0.81 (0.42–1.58), p = 0.55, respectively]. However, IFN-stayers showed increased risk of relapses compared with FTY-stayers [HR (95% CI) 1.46 (1.00–2.12), p = 0.05].
Conclusion
The ideal treatment option for MS is becoming increasingly complex, with the need to balance benefit and risks. Our results suggest that starting with FTY affects the long-term disease outcome similarly to escalating from IFN-β1a to FTY.




Similar content being viewed by others
References
Freedman MS, Selchen D, Prat A, Giacomini PS (2018) Managing multiple sclerosis: treatment initiation, modification, and sequencing. Can J Neurol Sci 45(5):489–503
Oh J, Vidal-Jordana A, Montalban X (2018) Multiple sclerosis: clinical aspects. Curr Opin Neurol 31(6):752–759
Gross RH, Corboy JR (2019) Monitoring, switching, and stopping multiple sclerosis disease-modifying therapies. Continuum (Minneap Minn) 25(3):715–735
Sormani MP, Bruzzi P (2015) Can we measure long-term treatment effects in multiple sclerosis? Nat Rev Neurol 11(3):176–182
Signori A, Gallo F, Bovis F et al (2016) Long-term impact of interferon or Glatiramer acetate in multiple sclerosis: a systematic review and meta-analysis. Mult Scler Relat Disord 6:57–63
Kappos L, Kuhle J, Multanen J et al (2015) Factors influencing long-term outcomes in relapsing-remitting multiple sclerosis: PRISMS-15. J Neurol Neurosurg Psychiatry 86:1202–1207
Mendes D, Alves C, Batel-Marques F (2016) Benefit-risk of therapies for relapsing–remitting multiple sclerosis: testing the number needed to treat to benefit (NNTB), number needed to treat to harm (NNTH) and the likelihood to be helped or harmed (LHH): a systematic review and meta-analysis. CNS Drugs 30:909–929
Cohen JA, Barkhof F, Comi G et al (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362:402–415
Kappos L, Radue EW, O'Connor P et al (2010) A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 362:387–401
Kappos L, O'Connor P, Radue EW et al (2015) Long-term effects of fingolimod in multiple sclerosis: the randomized FREEDOMS extension trial. Neurology 84:1582–1591
Cohen JA, Khatri B, Barkhof F et al (2016) Long-term (up to 4.5 years) treatment with fingolimod in multiple sclerosis: results from the extension of the randomised TRANSFORMS study. J Neurol Neurosurg Psychiatry 87:468–475
Trojano M, Bergamaschi R, Amato MP et al (2019) The Italian multiple sclerosis register. Neurol Sci 40:155–165
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302
Pardo G, Jones DE (2017) The sequence of disease-modifying therapies in relapsing multiple sclerosis: safety and immunologic considerations. J Neurol 264:2351–2374
Montalban X, Gold R, Thompson AJ et al (2018) ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler 24:96–120
Rae-Grant A, Day GS, Marrie RA et al (2018) Practice guideline recommendations summary: disease- modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 90:777–788
Comi G, Radaelli M, Soelberg Sørensen P (2017) Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet 389:1347–1356
Corboy JR, Weinshenker BG, Wingerchuk DM (2018) Comment on 2018 American Academy of Neurology guidelines on disease- modifying therapies in MS. Neurology 90:1106–1112
Rio J, Castilló J, Rovira A et al (2009) Measures in the first year of therapy predict the response to interferon beta in MS. Mult Scler 15:848–853
Sormani MP, Rio J, Tintorè M et al (2013) Scoring treatment response in patients with relapsing multiple sclerosis. Mult Scler 19:605–612
Lee S, Baxter DC, Limone B et al (2012) Cost-effectiveness of fingolimod versus interferon beta-1a for relapsing remitting multiple sclerosis in the United States. J Med Econ 15:1088–1096
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
G. Lucisano, A. Manni, S. Bonavita, V. Brescia Morra, A. Conte, F. De Robertis, P. Gazzola, A. Iaffaldano, C. Gasperini, M. Gatto, G.T. Maniscalco, I. Pesci report no disclosures relevant to the manuscript. D. Paolicelli received advisory board membership, speakers honoraria, travel support, research grants, consulting fees, or clinical trial support from Almirall, Bayer Schering, Biogen, Celgene, Excemed, Genzyme, Merck, Mylan, Novartis, Sanofi, Roche, Teva. M.P. Amato has received research grants and honoraria as a speaker and member of advisory boards by Biogen, Merck, Sanofi Genzyme, Teva, Novartis, Roche. C. Avolio served on scientific advisory boards for Merck, Sanofi-Genzyme and Roche, and has received travel and/or speaker honoraria from Merck, Roche, Teva, Biogen, Novartis and Sanofi-Genzyme. M. Capobianco received personal compensation for speaking at meeting or participating in advisory board from Almirall, Biogen, Merck, Novartis, Sanofi, Roche. E. Cocco has served on scientific Advisory Boards for Bayer, Biogen, Merck, Novartis, Roche, Sanofi Genzyme and TEVA; she also received speaker honoraria and research grants for her department by the same companies. G. Comi received personal compensation for consulting services and/or speaking activities from Novartis, Teva, Sanofi, Sanofi-Genzyme, Merck, Biogen, Excemed, Serono Symposia International Foundation, Roche, Almirall, Receptos, Celgene, and Forward Pharma. G. De Luca served on scientific advisory boards for Merck, Sanofi-Genzyme and Roche, and has received travel and/or speaker honoraria from Merck, Roche, Teva, Biogen, Novartis and Sanofi-Genzyme. G. Lus receveid travel fundings, research support, speaker honoraria from: Biogen, Novartis, Sanofi Genzyme, Bayer, Teva, Almirall, Allergan, Ipsen, Merck, Merz Pharma Italia. P. Iaffaldano received advisory board membership, speaker honoraria, travel support, research grants, consulting fees, or clinical trial support from Almirall, Bayer Schering, Biogen, Celgene, Excemed, Genzyme, Merck, Mylan, Novartis, Sanofi, Roche, Teva. D. Maimone receveid travel funds and speaker honoraria from Biogen, Novartis, Sanofi Genzyme, Teva, Almirall, Merck, Roche. G. Mallucci received support to travel to scientific meetings from Bayer Schering, Biogen, Genzyme, Merck Serono, Novartis, Roche, Sanofi-Aventis, Teva; received research grants for the MS Center at IRCCS Mondino, Pavia, Italy from Biogen and served on the scientific advisory board for Biogen, Genzyme and Merck Serono. G.A. Marfia is an Advisory Board member of Biogen Idec, Genzyme, Merck-Serono, Novartis, Teva and received honoraria for speaking or consultation fees from Almirall, Bayer Schering, Biogen Idec, Merck Serono, Novartis, Sanofi-Genzyme, Teva. She is the principal investigator in clinical trials for Actelion, Biogen Idec, Merck Serono, Mitsubishi, Novartis, Roche, Sanofi-Genzyme, Teva. F. Patti has received honoraria for speaking activities by Bayer Schering, Biogen, Merck, Novartis, Roche, TEVA and Sanofi Aventis; he also served as advisory board member the following companies: Bayer Schering, Roche, Biogen, Merck, Novartis; he was also funded by Pfizer and FISM for epidemiological studies; finally he received grant for congress participation from Bayer Schering, Roche, Biogen, Merck, Novartis, Sanofi Aventis, and TEVA. C. Pozzilli has received consultant fees from Actelion, Biogen, Genzyme, Merck-Serono, Novartis, Teva Neurosciences and grant or research support from Biogen, Merck-serono, Novartis and Teva neuroscinces. M. Rovaris received fees for consulting or scientific speaking from Almirall, Biogen, Genzyme-Sanofi, Merck Serono and TEVA. G. Salemi received grants and honoraria by Almirall, Biogen-Dompè, Merck-Serono, Novartis, Roche, Sanofi-Aventis, and TEVA. M. Salvetti received research support and consulting fees from Biogen, Merck, Novartis, Roche, Sanofi, Teva. D. Spitaleri received fees for consulting or congress participation from Novartis, TEVA, Sanofi and Biogen. R. Totaro has served on advisory boards and/or received honoraria for speaking or consultation fees from Biogen, CSL behring, Merck-Serono, Novartis, Roche, Sanofi-Genzyme, Shire, Teva. M. Zaffaroni received personal financial support for attending scientific meetings from Merck and financial support for his Department from Novartis. M. Trojano received advisory board membership, speaker honoraria, travel support, research grants, consulting fees, or clinical trial support from Actelion, Allergan, Almirall, Bayer Schering, Biogen, Celgene, Excemed, Genzyme, Merck, Mylan, Novartis, Sanofi, Roche, Teva.
Ethic approval
The study was conducted in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975. The study was approved by the Ethic Committee of AOU Policlinico, Bari on 17 July 2018; ID number 0060669.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paolicelli, D., Lucisano, G., Manni, A. et al. Retrospectively acquired cohort study to evaluate the long-term impact of two different treatment strategies on disability outcomes in patients with relapsing multiple sclerosis (RE.LO.DI.MS): data from the Italian MS Register. J Neurol 266, 3098–3107 (2019). https://doi.org/10.1007/s00415-019-09531-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09531-6