Abstract
Background
No postmarketing randomised clinical trials are available about alemtuzumab, and real-world data are limited. We aimed to analyse the efficacy and safety of alemtuzumab in a single-centre cohort of patients with relapsing–remitting MS.
Methods
Patients who took alemtuzumab were enrolled. We collected the following data: age, sex, MS history, expanded disability status scale (EDSS), relapses, magnetic resonance imaging (MRI) parameters after alemtuzumab, and adverse events. EDSS scores before alemtuzumab and at the last follow-up were compared by Wilcoxon test. Time to first relapse was analysed after dividing the cohort on the basis of previous treatment.
Results
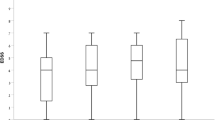
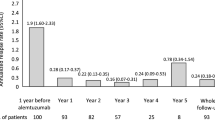
Ninety patients were enrolled [women 74.4%; naïve 7; mean follow-up 27 months (SD 23)]. The EDSS was reduced from a median of 2.5 (IQR 1.5–4) before alemtuzumab to 2.0 (IQR 1.5–3.5) after (p = 0.025). The time to first relapse was shorter in patients shifting from a second-line therapy (p = 0.011). Over 2 years, 43.7% had no evidence of disease activity. We observed infusion-related reactions in 95.5% patients, including 11.1% with pneumonitis, thyroiditis in 11%, and thrombocytopenia in 3.3%.
Conclusions
We confirmed the clinical and MRI efficacy of alemtuzumab in the clinical setting and the frequency of infusion-related reactions. Compared with that in clinical trials, higher number of patients developed pneumonitis during infusion.

Similar content being viewed by others
References
Thompson AJ, Baranzini SE, Geurts J et al (2018) Multiple sclerosis. Lancet 391(10130):1622–1636
Montalban X, Belachew S, Wolinsky JS (2017) Ocrelizumab in primary progressive and relapsing multiple sclerosis. N Engl J Med 376(17):1694
CAMMS223 Trial Investigators, Coles AJ, Compston DA, Selmaj KW et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359(17):1786–1801
Cohen JA, Coles AJ, Arnold DL et al (2012) Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 380(9856):1819–1828
Coles AJ, Twyman CL, Arnold DL et al (2012) Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 380(9856):1829–1839
Havrdova E, Arnold DL, Cohen JA et al (2017) Alemtuzumab CARE-MS I 5-year follow-up: durable efficacy in the absence of continuous MS therapy. Neurology 89(11):1107–1116
Coles AJ, Cohen JA, Fox EJ et al (2017) Alemtuzumab CARE-MS II 5-year follow-up: efficacy and safety findings. Neurology 89(11):1117–1126
Willis MD, Robertson NP (2016) Alemtuzumab for multiple sclerosis. Curr Neurol Neurosci Rep 16(9):84
Gerardi C, Bertele’ V, Rossi S et al (2018) Preapproval and postapproval evidence on drugs for multiple sclerosis. Neurology 90(21):964–973
Ziemssen T, Thomas K (2017) Alemtuzumab in the long-term treatment of relapsing-remitting multiple sclerosis: an update on the clinical trial evidence and data from the real world. Ther Adv Neurol Disord 10(10):343–359
Maniscalco GT, Cerillo I, Servillo G et al (2018) Early neutropenia with thrombocytopenia following alemtuzumab treatment for multiple sclerosis: case report and review of literature. Clin Neurol Neurosurg 175:134–136
Hoffman BM, Zeid NA, Alam U et al (2019) Lambert–Eaton myasthenic syndrome associated with alemtuzumab administration. Mult Scler Relat Disord 27:131–132
Ranganathan U, Kaunzner U, Foster S et al (2018) Immediate transient thrombocytopenia at the time of alemtuzumab infusion in multiple sclerosis. Mult Scler 24(4):540–542
Madeley J, Hodges G, Birchley A. Development of acquired haemophilia A in a patient treated with alemtuzumab for multiple sclerosis. BMJ Case Rep. 2018;2018: bcr-2018
Haghikia A, Dendrou CA, Schneider R et al (2017) Severe B-cell-mediated CNS disease secondary to alemtuzumab therapy. Lancet Neurol 16(2):104–106
Wehrum T, Beume LA, Stich O et al (2018) Activation of disease during therapy with alemtuzumab in 3 patients with multiple sclerosis. Neurology 90(7):e601–e605
Bernard-Valnet R, Pignolet B, Biotti D et al (2018) Unexpected high multiple sclerosis activity after switching from fingolimod to alemtuzumab. Mult Scler Relat Disord 25:216–218
Willis M, Pearson O, Illes Z et al (2017) An observational study of alemtuzumab following fingolimod for multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 4(2):e320
Tuohy O, Costelloe L, Hill-Cawthorne G et al (2015) Alemtuzumab treatment of multiple sclerosis: long-term safety and efficacy. J Neurol Neurosurg Psychiatry 86(2):208–215
Prosperini L, Annovazzi P, Boffa L et al (2018) No evidence of disease activity (NEDA-3) and disability improvement after alemtuzumab treatment for multiple sclerosis: a 36-month real-world study. J Neurol 265(12):2851–2860
Huhn K, Bayas A, Doerck S et al (2018) Alemtuzumab as rescue therapy in a cohort of 50 relapsing-remitting MS patients with breakthrough disease on fingolimod: a multi-center observational study. J Neurol 265(7):1521–1527
Parks NE, Flanagan EP, Lucchinetti CF et al (2017) NEDA treatment target? No evident disease activity as an actionable outcome in practice. J Neurol Sci 383:31–34
Clerico M, De Mercanti S, Artusi CA et al (2017) Active CMV infection in two patients with multiple sclerosis treated with alemtuzumab. Mult Scler 23(6):874–876
Hatcher SE, Waubant E, Nourbakhsh B et al (2016) Rebound syndrome in patients with multiple sclerosis after cessation of fingolimod treatment. JAMA Neurol 73(7):790–794
Frau J, Sormani MP, Signori A et al (2018) Clinical activity after fingolimod cessation: disease reactivation or rebound? Eur J Neurol 25(10):1270–1275
Wiendl H, Calabresi PA, Meuth SG (2018) Defining response profiles after alemtuzumab: rare paradoxical disease exacerbation. Neurology 90(7):309–311
Sheikh-Taha M, Corman LC (2017) Pulmonary Nocardia beijingensis infection associated with the use of alemtuzumab in a patient with multiple sclerosis. Mult Scler 23(6):872–874
Blasco MR, Ramos A, Malo CG et al (2017) Acute pneumonitis and pericarditis related to alemtuzumab therapy in relapsing-remitting multiple sclerosis. J Neurol 264(1):168–169
Sardu C, Cocco E, Mereu A et al (2012) Population based study of 12 autoimmune diseases in Sardinia, Italy: prevalence and comorbidity. PLoS One 7(3):e32487
Devonshire V, Phillips R, Wass H et al (2018) Monitoring and management of autoimmunity in multiple sclerosis patients treated with alemtuzumab: practical recommendations. J Neurol 265(11):2494–2505
Kalincik T, Brown JWL, Robertson N et al (2017) Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study. Lancet Neurol 16(4):271–281
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interests about this work.
Rights and permissions
About this article
Cite this article
Frau, J., Coghe, G., Lorefice, L. et al. Efficacy and safety of alemtuzumab in a real-life cohort of patients with multiple sclerosis. J Neurol 266, 1405–1411 (2019). https://doi.org/10.1007/s00415-019-09272-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09272-6